Optimizing Nursing Management of Patients Receiving
advertisement

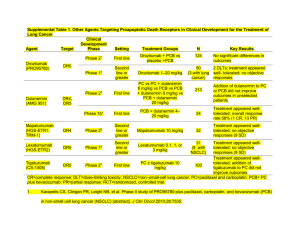
Optimizing Nursing Management of Patients Receiving Novel Therapies for Advanced NSCLC Beth Eaby-Sandy, MSN, CRNP, OCN® Nurse Practitioner Abramson Cancer Center of the University of Pennsylvania Disclosure of Conflicts of Interest Beth Eaby-Sandy, MSN, CRNP, OCN® discloses she was a member of the advisory board of Boehringer Ingelheim and a member of the speakers’ bureau of Genentech in the last 12 months. She is currently a member of the speakers’ bureau of Lilly. NSCLC: Scope of the Problem Estimated New Cancer Cases in 2013 • Prostate: 238,590 (28%) • Lung/Bronchus: 118,080 (14%) • Colon/Rectum: 73,680 (9%) • Breast: 226,870 (29%) • Lung/Bronchus: 110,110 (14%) • Colon/Rectum: 69,140 (9%) ACS, 2013. NSCLC: Scope of the Problem Estimated New Cancer Cases in 2013 • Prostate: 238,590 (28%) • Lung/Bronchus: 118,080 (14%) • Colon/Rectum: 73,680 (9%) • Breast: 226,870 (29%) • Lung/Bronchus: 110,110 (14%) • Colon/Rectum: 69,140 (9%) PERSPECTIVE Lung/Bronchus Deaths = 159,480 Breast, Prostate, Colon/Rectum, and Pancreatic Deaths = 158,630 ACS, 2013. Lung Cancer Stages and Survival 2003-2009, All Races, Both Sexes Stage Distribution (%) 5-Year Relative Survival (%) 60 Percent 50 40 30 20 10 0 Localized (confined to primary Regional (spread to regional site) lymph nodes) Stage SEER Data, 2003-2009. Distant (cancer has metastasized) Unknown (unstaged) NSCLC: Breakdown by Subtype Squamous (30-35%) – Usually more centralized – More frequently associated with significant cough and hemoptysis Eaby-Sandy, 2011. NSCLC: Breakdown by Subtype Nonsquamous (65%) – Adenocarcinoma (40%) • Most common type of NSCLC • Most common type in nonsmokers – Large cell carcinoma (15%) – Mixed or NOS (10%) NOS = not otherwise specified. Eaby-Sandy, 2011. NSCLC: Why Does Histology Matter? In past, all NSCLC patients treated the same Data have shown that the use of certain agents in certain histologies results in improved survival and response rates Histology may predict the presence of biomarkers Safety parameters of certain treatments depend on histology NCCN, 2013. NSCLC: Breakdown of Biomarkers 1% 3% 3% Unknown KRAS 3% 2% EGFR 5% ALK RET 5% MET 33% PI3K BRAF 15% HER2 PDGFR VEGFR 25% FGFR1 AKT1 MEK1 ROS1 Hirsch, 2012. Molecular Abnormalities in NSCLC With Current Implications EGFR • Transmembrane receptor • Detectable in about 80-85% of patients • Level of expression varies widely • Mutations in this domain (10-15% of pts) result in activation of the tyrosine kinase domain with significantly better response to erlotinib or gefitinib • Mutations: highest incidence in never smokers, adenocarcinoma, women, and patients with Asian ethnicity KRAS • • • • EML4-ALK • Incidence of EML4-ALK translocation: 2-7% • Estimated prevalence of EML4-ALK in lung cancer: 6,000 pts/yr US; up to 40,000 pts/yr globally • Most EML4-ALK fusion events observed in lung adenocarcinoma specimens vs squamous or small cell histologies • EML4-ALK rarely coexists with EGFR, HER2, or KRAS mutations, indicating it is a distinct disease subtype 25% of North American population Associated with smoking and resistance to tyrosine kinase inhibitors KRAS mutations associated with shorter survival Therapy with drugs other than erlotinib should be considered first NCCN, 2013; Langer et al, 2010. Why Do Biomarkers Matter? PFS with standard chemotherapy regimens in NSCLC: – Pemetrexed/cisplatin: 5 months – Paclitaxel/carboplatin/bevacizumab: 6 months PFS with EGFR mutated NSCLC receiving EGFR targeted therapy: – Gefitinib: 9.5 months – Erlotinib: 9.7 months – Afatinib: 11.0 months PFS with ALK-positive NSCLC receiving ALK inhibitor: – Crizotinib: 9.7 months PFS = progression-free survival. Camidge et al, 2012; Yang, Shih et al, 2012; Rosell et al, 2012; Fukuoka et al, 2011; Scagliotti et al, 2008; Sandler et al, 2006. Patient Factors in Treatment Planning Patient ECOG PS – PS is a predictor of survival/tolerating chemotherapy – PS 0/1 patients tolerate chemotherapy best – PS 2 patients can potentially benefit, even from doublet chemotherapy; however, toxicity must be monitored closely Comorbidities – Diabetes, heart disease – Renal disease ECOG PS = Eastern Cooperative Oncology Group performance status. Rodriguez & Lilenbaum, 2008. Patient Factors in Treatment Planning Patient goals for treatment – Quality-of-life issue (eg, hair loss) – Advanced directives Demographics Social support – Involve social worker – Counseling services – Nutrition services Financial issues Evolving Supportive Care Paradigms Early palliative care leads to increase in OS in patients with metastatic NSCLC Increased quality of life, less depressive symptoms Improved understanding of diagnosis – 1/3 patients at diagnosis thought they had curable disease – Less likely to receive chemotherapy near end of life OS = overall survival. Temel et al, 2011; Temel et al, 2010. Case Study: First-Line Treatment Mrs. JF: History 68-year-old woman, presented 1 month ago with pain in her lower back Initial management with NSAIDs somewhat helpful; however, the pain persisted and an x-ray of the lower spine was ordered X-ray did not show bone abnormality but revealed a right lung mass at the right lung base Further imaging with PET/CT revealed a right lung mass, mediastinal lymphadenopathy, bone metastases in the lumbar spine, and liver metastases – X-rays are often negative NSAIDs = nonsteroidal antiinflammatory drugs; PET/CT = positron emission tomography/computed tomography. Mrs. JF: Diagnostic Evaluation Treating physician referred patient to pulmonologist for a bronchoscopy with biopsy – Mediastinal lymph node was positive for NSCLC, adenocarcinoma histology MRI scan of the brain negative for metastatic disease Baseline labs within normal limits Baseline PS 1 What factors important in treatment planning? MRI = magnetic resonance imaging. Mrs. JF: Treatment Considerations Bronchoscopy yielded core biopsy, able to perform KRAS, EGFR, ALK, and ROS testing. All were negative. Patient has a 45-pack-year smoking history, currently trying to quit Patient has hypertension (controlled with medication), hypercholesterolemia, and chronic obstructive pulmonary disease No significant weight loss, no hemoptysis Understands incurable, no advanced directive, would like to “fight” Mrs. JF: Treatment Selection Standard chemotherapy in biomarker-negative patient appropriate therapy Numerous options available for chemotherapy Platinum based chemotherapy appropriate given good PS Is hair loss an issue? Does she want to enroll in a clinical trial? NCCN, 2013. Mrs. JF: Treatment Selection Cisplatin versus carboplatin? In US, often use carboplatin in frontline therapy What drug to pair with carboplatin? Toxicity? – Pemetrexed – Paclitaxel – Docetaxel – nab-paclitaxel – Gemcitabine – Vinorelbine ECOG 1594: All Platinum Doublets Essentially Equal 1,207 patients, stage IIIB/IV (15/85%), PS 0-2 Median age 63; male/female: 64/36% R A N D O M I Z E Cisplatin Paclitaxel 75 mg/m2 D2 135 mg/m2/24h q3w Cisplatin Gemcitabine 100 mg/m2 D1 1 g/m2 D1,8,15 q4w Cisplatin Docetaxel 75 mg/m2 D1 75 mg/m2 D1 q3w Carboplatin Paclitaxel AUC=6 mg/mL/min D1 q3w 225 mg/m2/3h D1 AUC = area under the curve. Schiller et al, 2002. Similar efficacy for all doublets Importance of Histology Overall Survival for Pemetrexed/Cisplatin Versus Gemcitabine/Cisplatin in First-Line Adenocarcinoma Patients Median OS (months) (95% CI) CI = confidence interval. Scagliotti et al, 2008; Scagliotti & Novello, 2003. Pemetrexed /Cisplatin Gemcitabine /Cisplatin (n=436) (n=411) 12.6 (10.7-13.6) 10.9 (10.2-11.9) What About Bevacizumab? Targeted therapy that can be added to chemotherapy in metastatic NSCLC Eligibility criteria and warnings: – – – – – – – – – – – Nonsquamous histology only No history hemoptysis (postprocedure ok?) No recent history of arterial thrombotic event No uncontrolled hypertension Nephrotic syndrome (proteinuria ≥3.5gm) No surgery within 28 days Gastrointestinal perforation Non-gastrointestinal fistula formation Reversible posterior leukoencephalopathy syndrome Infusion reactions Ovarian failure Avastin® prescribing information, 2013. E4599 Trial: Bevacizumab + PC Versus PC Alone in First-Line Nonsquamous NSCLC 1-year survival: 51% vs 44% 2-year survival: 23% vs 15% PC = paclitaxel + carboplatin. Sandler et al, 2006; Sandler et al, 2011. Median OS with Bevacizumab + PC was 12.3 months vs 10.3 months for PC alone (P=0.013) PointBreak Trial: Can Regimens Be Combined? Randomized, open-label, phase III superiority study conducted in US Pemetrexed 500 mg/m2; carboplatin AUC 6; bevacizumab 15 mg/kg Paclitaxel 200 mg/m2; carboplatin AUC 6; bevacizumab 15 mg/kg Inclusion: − No prior systemic therapy for lung cancer − PS 0/1 − Stage IIIB-IV nonsquamous NSCLC − Stable treated brain metastasized R 1:1 Exclusion: − Peripheral neuropathy ≥grade 1 − Uncontrolled pleural effusions q21d= every 21 days; PD = progressive disease. Patel et al, 2012. Induction Phase q21d, 4 cycles Maintenance Phase q21d until PD Pemetrexed + Carboplatin + Bevacizumab Pemetrexed + Bevacizumab 450 Patients Each Paclitaxel + Carboplatin + Bevacizumab Bevacizumab PointBreak Trial: OS Did Not Differ Between Treatment Arms PointBreak: KM OS From Randomization (ITT) ITT = intent to treat; KM = Kaplan-Meier. Patel et al, 2012. Which Regimen to Choose for First-Line Treatment? Discuss toxicity profiles of different regimens Take histology into account Give patients autonomy to decide – Do they want treatment? – If so, which regimen’s toxicity profile is right for them? – Comorbidities? Diabetes? Coronary artery disease? Renal insufficiency? Maintenance Therapy If no disease progression after first-line chemotherapy, continue the chemotherapy and/or targeted agent? We know that in NSCLC continuing platinum chemotherapy past 4 to 6 cycles does not improve survival, just increases toxicity However, three drugs have shown improvement in PFS in the maintenance setting NCCN, 2013. E4599 Trial: Bevacizumab + PC Versus PC Alone in First-Line Nonsquamous NSCLC First-line treatment of patients with stage IIIB and malignant pleural effusion, stage IV, or recurrent NSCLC (N=878) Stratified by • Disease stage • Degree of weight loss • Prior radiotherapy • Measurable disease q3w = every 3 weeks; IV = intravenously. Sandler et al, 2006. Paclitaxel 200 mg/m2 + carboplatin AUC=6 q3w x 6 (no crossover permitted) (n=444) Bev 15 mg/kg Solution for IV infusion q3w + PC x 6 (n=434) Continued until progression or unacceptable toxicity Bev 15 mg/kg IV q3w until disease progression or unacceptable toxicity Primary end point OS Secondary end points Response rate, PFS, toxicity Maintenance Pemetrexed Both studies showed improvement in OS when either switching or continuing on with maintenance pemetrexed after first-line induction platinum-based chemotherapy. Ciuleanu et al, 2009; Paz-Ares et al, 2012. PointBreak Trial: Data Did Not Favor Either Maintenance Regimen Randomized, open-label, phase III superiority study conducted in US Pemetrexed 500 mg/m2; carboplatin AUC 6; bevacizumab 15 mg/kg Paclitaxel 200 mg/m2; carboplatin AUC 6; bevacizumab 15 mg/kg Inclusion: − No prior systemic therapy for lung cancer − PS 0/1 − Stage IIIB-IV nonsquamous NSCLC − Stable treated brain metastasized Exclusion: − Peripheral neuropathy ≥grade 1 − Uncontrolled pleural effusions Patel et al, 2012. R 1:1 Induction Phase q21d, 4 cycles Maintenance Phase q21d until PD Pemetrexed + Carboplatin + Bevacizumab Pemetrexed + Bevacizumab 450 Patients Each Paclitaxel + Carboplatin + Bevacizumab Bevacizumab SATURN Trial: Erlotinib Maintenance Chemotherapy-naive, advanced NSCLC N=1,949 4 cycles of first-line platinum-based doublet Non-PD n=889 Mandatory tumor sampling SATURN included patients with the following tumor types: – Squamous cell carcinoma – Nonsquamous cell carcinoma (adenocarcinoma, large cell, other) Coprimary end points: – PFS in all patients – PFS in patients with EGFR IHC-positive tumors Secondary end points: – OS in all patients and those with EGFR IHC-positive tumors – OS and PFS in EGFR IHC-negative tumors – Safety IHC = immunohistochemistry. Cappuzzo et al, 2010. Erlotinib 150 mg/d n=438 Placebo n=451 PD PD SATURN Trial: Erlotinib Maintenance 19% reduction in risk of death OS in a broad ITT population OS rates in the SATURN ITT population at milestone Erlotinib (n=438) 1.0 Overall Survival Probability Placebo (n=451) 0.8 HR=0.81 95% CI:0.70-0.95; P=0.0088 0.6 Median 12.0 months with erlotinib vs 11.0 months with placebo 0.4 0.2 11.0 months median OS 12.0 months median OS 0 0 3 HR = hazard ratio. Cappuzzo et al, 2010. 6 9 12 15 18 21 Time (months) 24 27 30 33 36 Maintenance Treatment Conclusions Again, there are options, just like first-line chemotherapy choice Do patients want a break or wish to continue? Toxicity profile – Pemetrexed is chemotherapy: potential for lowering of blood counts, requires vitamin supplementation – Bevacizumab and erlotinib are targeted agents, with the potential for hypertension/cardiac toxicity, rash Cost? Should this be an issue? Insurance coverage/denials? Case Study: Biomarker-Positive Patient Ms. LT: History 47-year-old woman who had a cough for 2 months. Antibiotics and antihistamines did not improve her symptoms. Chest x-ray revealed multiple small masses in bilateral lungs and pleural effusion, confirmed by CT; PET/CT revealed no other disease outside of chest MRI scan of brain negative for metastatic disease Ms. LT: Diagnostic Evaluation Pleural fluid positive for adenocarcinoma histology; however, not enough for molecular analysis Bronchoscopy/endobronchial ultrasound performed; able to biopsy mediastinal lymph node to get more tissue Molecular testing revealed an exon 19 deletion EGFR mutation Patient is a never smoker with no significant medical history or comorbidities EGFR-Positive NSCLC: CT Chest Scan at Diagnosis Image courtesy of Beth Eaby-Sandy, MSN, CRNP, OCN® Ms. LT: Treatment Considerations Chemotherapy versus targeted therapy in first-line treatment? How long did it take to receive the molecular testing results? Toxicity Current profiles, how do they differ? approved EGFR tyrosine kinase inhibitors: gefitinib (no longer approved in US), erlotinib, and afatinib EURTAC Trial First-Line Erlotinib Versus Chemotherapy in EGFR Mutation-Positive NSCLC Patients Rosell et al, 2012. Another EGFR Inhibitor Afatinib – Irreversible pan-EGFR/HER inhibitor • Approved in July 2013 for first-line treatment of EGFR mutation-positive metastatic NSCLC – Dose is 40 mg daily, orally Yang, Shih et al, 2012. Primary End Point: PFS Independent review ‒ All Randomly Assigned Patients Progression-Free Survival (probability) 1.0 Afatinib n=230 Cis/pem n=115 PFS event, n (%) 152 (66) 0.8 Median PFS (months) 11.1 HR (95% CI) 0.6 69 (60) 6.9 0.58 (0.43–0.78) P=0.0004 47% 0.4 0.2 22% 0.0 0 Number at risk Afatinib 230 Cis/Pem 115 3 6 9 12 15 18 21 24 27 10 2 3 0 0 0 Progression-Free Survival (months) 180 72 151 41 Yang, Schuler et al, 2012; Yang, Shih et al, 2012. 120 21 77 11 50 7 31 3 Ms. LT: Treatment Selection The patient is treated with erlotinib, first line Dose is 150 mg daily on an empty stomach Most common toxicities in erlotinib arm: – Papulopustular rash: 80% (grade 3= 13%) – Diarrhea: 57% (grade 3=5%) – Fatigue: 57% (grade 3=6%) – Anorexia 31% (grade 3=0%) Rosell et al, 2012. Incidence and Severity of Rash Drug All Rash Incidence Grade 3/4 Incidence Cetuximab 89% (70% in FLEX trial) 12% (10% in FLEX trial) Erlotinib 75% 9% Gefitinib Rash: 43% Acne: 25% 0% (only reported ≥5%) 0% Panitumumab 89% 12% Afatinib 89% 16% Erbitux® prescribing information, 2013; Tarceva® prescribing information, 2010; Iressa® prescribing information, 2010; Vectibix® prescribing information, 2013; Yang, Shih et al, 2012. Strategies to Prevent Dermatologic Toxicities: Pre-Emptive STEPP in metastatic colorectal cancer patients who received panitumumabcontaining regimens 95 total patients: – Significant improvement in EGFR rash and quality of life with pre-emptive doxycycline and topical hydrocortisone cream. – At 6 weeks, grade ≥2 skin toxicities were reduced by more than 50% in the pre-emptive arm STEPP = Skin Toxicity Evaluation Protocol With Panitumumab. Lacouture et al, 2010. MASCC Rash Prevention and Treatment Guidelines Preventive (Weeks 1-6,8 of EGFR Inhibitor initiation) Recommend Not Recommended Level of Evidence Recommendation Grades Comments Topical Hydrocortisone 1% cream with moisturizer and sunscreen BID • Pimecrolimus 1% cream • Tazarotene 0.05% cream • Sunscreen as single agent IIa C Doxycycline is preferred in patients with renal impairment. Minocycline is less photosensitizing. Systemic • Minocycline 100 mg daily • Doxycyline 100 mg BID Tetracycline 550 mg BID IIa A Topical • Alclometasone 0.05% cream • Fluocinonide 0.05% cream BID • Clindamycin 1% Vitamin K1 Cream IVa C Fluocinonide 0.05% cream BID should not be used on the face for more than 2 weeks at a time. Systemic • Doxycycline 100 mg BID • Minocycline 100 mg daily • Isotretinoin at low doses (20-30 mg/d) Acitretin IVa C Isotretinoin is photosensitizing and can cause xerosis. Monitor lipids and liver enzymes with retinoids. aEGFR inhibitor study. MASCC = Multinational Association of Supportive Care in Cancer. BID = twice daily. Lacouture et al, 2011. Mild Rash Image courtesy of Beth Eaby-Sandy, MSN, CRNP, OCN® Moderate Rash Image courtesy of Beth Eaby-Sandy, MSN, CRNP, OCN® Severe Rash Image courtesy of Beth Eaby-Sandy, MSN, CRNP, OCN® Other Cutaneous Toxicities Alopecia/scalp rash Paronychia Hypertrichosis Fissures Images courtesy of Beth Eaby-Sandy, MSN, CRNP, OCN® Case Study: Older Adult With NSCLC Mr. PD: History Patient is an 80-year-old fit man who developed increased shortness of breath and cough during the past 6 months, though hemoptysis is what led him to the emergency department CT scan of the chest reveals a large, central lung mass as well as adrenal metastases He is a lifelong cigarette smoker, 1 pack per day CT-guided needle biopsy reveals squamous cell NSCLC Mr. PD: Diagnostic Evaluation Brain MRI scan shows a single brain metastasis 1.5 cm, for which he undergoes stereotactic brain radiation Patient presents to oncology office to decide about treatment options for his cancer Patient has a supportive wife and daughter; he still plays golf once a week and bridge with his friends on Wednesday nights Incidence of NSCLC in the US by Age at Diagnosis Langer et al, 2010; SEER Data 1975-2002. Mr. PD: Treatment Considerations Chemotherapy has survival advantage over best supportive care for the fit elderly Patient would like to maintain ability to play golf and bridge and spend time with grandchildren Chemotherapy with platinum-based doublet is an option for him What can we give him that can maintain quality of life and yet give him chance for increased survival? Family wants him to pursue treatment. NCCN, 2013. IFCT-0501: Weekly PC Doublet Superior to Single-Agent Chemotherapy Overall survival (ITT) MST = median survival time. Quoix et al, 2011. Which Treatment Regimen to Use? Toxicity was similar in both arms Weekly paclitaxel/carboplatin a reasonable treatment option for elderly patients based on 2011 trial data More recent study examined weekly nab-paclitaxel/carboplatin versus paclitaxel/carboplatin every 3 weeks Quoix et al, 2011. nab-Paclitaxel in Elderly Patients • In elderly patients, a nonsignificant trend toward improved PFS (8.0 vs 6.8 months; HR=0.687; 95% CI:0.420-1.123; P=0.134) • A significant improvement in OS was observed with nab-PC vs sb-PC • In patients <70 years of age, there was no difference in PFS or OS Kaplan-Meier Curve of Overall Survival in Patients ≥70 Years Probability of Survival 1.00 nab-PC (n=74) sb-PC (n=82) 0.75 19.9 months 0.50 10.4 months 0.25 HR=0.583 95% Cl:0.388-0.875 P=0.009 0.00 0 3 6 9 12 Months sb-PC = subcutaneous paclitaxel. Socinski et al, 2013. 15 18 21 24 Populations That Benefited Most • North America • Elderly (age ≥70) • Squamous histology Socinski et al, 2013. Neuropathy in Elderly Patients in nab-Paclitaxel Study FACT-Taxane Results in Patients ≥70 Years 6 Peripheral Neuropathy 4.99 5 4 3 1.86 2 1 0 BL C2 Socinski et al, 2013. C3 C4 C5 C6 C7 C8 Final FACT Subscore: Mean Baseline Score or Mean Change from Baseline FACT Subscore: Mean Baseline Score or Mean Change From Baseline nab-PC 3 sb-PC Pain in Hands/Feet 2.19 2.5 2 1.5 0.70 1 0.5 0 -0.5 BL C2 C3 C4 C5 C6 C7 C8 Final Management of CIPN Complicating comorbidities, are they under control? Assessment: FACT-Taxane? DTRs? Vibration testing? Neurological consult for electromyography? Several studies evaluating agents such as nortriptyline, amitriptyline, gabapentin, and lamotrigine have not shown a benefit, though these agents are often used in clinical practice Duloxetine is the only agent shown to diminish CIPN in a phase III trial DTRs = deep tendon reflexes; CIPN = chemotherapy-induced peripheral neuropathy. Eaby-Sandy, 2013. Patient Education Challenges Explaining targeted therapy versus chemotherapy Adherence to oral therapies – Cost, Medicare “donut hole” – Over-adherence vs under-adherence – Increased clinic visits – Phone call support – Logging, pillboxes – Education on side-effect management Neuss et al, 2013. Key Takeaways Treatment strategies for advanced NSCLC continue to evolve: maintenance, more aggressive treatment for elderly patients Toxicity profiles can vary significantly depending on selected agent(s) Oncology nurses play an important role in monitoring for and managing toxicities, as well as providing patient education References Alimta® (pemetrexed) prescribing information (2012). Eli Lilly and Company. American Cancer Society (2013). American Cancer Society: Cancer facts & figures 2013. Atlanta. Avastin® (bevacizumab) prescribing information (2013). Genentech USA, Inc. Camidge DR, Bang YJ, Kwak EL, et al (2012). Activity and safety of crizotinib in patients with ALK-positive non-smallcell lung cancer: updated results from a phase 1 study. Lancet Oncol, 13(10):1011-1019. Cappuzzo F, Ciuleanu T, Stelmakh L, et al (2010). Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicenter, randomized, placebo-controlled phase 3 study. Lancet, 11(6):521-529. Capuzzo F, Marchetti A, Skokan M, et al (2009). Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol, 27(10):1667-1674. Ciuleanu T, Brodowicz T, Zielinski C, et al (2009). Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet, 374(9699):1432-1440. Eaby-Sandy B (2011). Cancer Nursing: Principals and Practice. Jones and Bartlett Publishers. Sudbury, Massachusetts. Eaby-Sandy B, Ko A, Renschler et al (2013). Efficacy and toxicity profile of nab-paclitaxel in patients with advanced non-small cell lung cancer (NSCLC): nursing implications and management strategies. Poster presented at Oncology Nursing Society 38th Congress in Washington, DC. Erbitux® (cetuximab) prescribing information (2013). New York, NY: ImClone Systems, Inc and Princeton, NJ: BristolMyers Squibb Co. Fukuoka M, Wu YL, Thongprasert S, et al (2011). Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol, 29(21):2866-2874. References Hirsch FR (2012). Recent advances in biomarker research in lung cancer with special reference to new targeted therapies. Presented at: 13th International Lung Cancer Congress; July 19-22, 2012; Huntington Beach, CA. Iressa® (gefitinib) prescribing information (2010). Wilmington, DE: AstraZeneca Pharmaceuticals LP. Lacouture ME, Anadkat MJ, Bensadoun RJ, et al (2011). Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer, 19(8):1079-1095. Lacouture ME, Mitchell EP, Piperdi B, et al (2010). Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol, 28(8):1351-1357. Langer CJ, Besse B, Gualberto A, et al (2010). The evolving role of histology in the management of advanced nonsmall cell lung cancer. J Clin Oncol, 28(36):5311-5320. National Comprehensive Cancer Network (NCCN) (2013). Clinical practice guidelines in oncology. Non-small cell lung cancer. V.2.2013. Available at http://www.nccn.org. Neuss MN, Polovich M, McNiff K, et al (2013). 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards including standards for the safe administration and management of oral chemotherapy. J Oncol Practice, 9(Suppl):5S-13S. Paz-Ares L, de Marinis F, Dediu M, et al (2012). Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol, 13(3):247-255. References Quoix E, Zalcman G, Oster JP, et al (2011). Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet, 378(9796):1079-1088. Rodriguez E, Lilenbaum RC (2008). New treatment strategies in patients with advanced non-small-cell lung cancer and performance status 2. Clin Lung Cancer, 9(6):326-330. Rosell R, Carcereny E, Gervais R, et al (2012). Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, openlabel, randomised phase 3 trial. Lancet Oncology, 13(3):239-246. Sandler A, Graham C, Baggstrom M, et al (2011). An open-label, multicenter, three-stage, phase II study of s-1 in combination with cisplatin as first-line therapy for patients with advanced non-small cell lung cancer. J Thoracic Oncol, 6(8):1400-1406. Sandler A, Gray R, Perry MC, et al (2006). Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med, 355(24):2542-2550. Patel JD, Socinski MA, Garon EB, et al (2012). A randomized, open-label, phase III, Label, Phase III, superiority study of pemetrexed (Pem) + carboplatin (Cb) + bevacizumab (Bev) followed by maintenance Pem + Bev versus paclitaxel (Pac) Cb + Bev followed by maintenance Bev in patients with sage IIIB or IV non-squamous non-cmall cell lung cancer (NS-NSCLC). Available at: http://www.thoracicsymposium.org/MeetingProgram/documents/PLPatel.pdf. Scagliotti GV, Novello S (2003). Pemetrexed and its emerging role in the treatment of thoracic malignancies. Expert Opin Investig Drugs, 12(5):853-863. References Scagliotti GV, Parikh P, von Pawel J, et al (2008). Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage nonsmall-cell lung cancer. J Clin Oncol, 20;26(21):3543-3551. Schiller JH, Harrington D, Belani CP, et al (2002). Comparison of four chemotherapy regimens for advanced non-smallcell lung cancer. N Engl J Med, 346(2):92-98. Socinski MA, Langer CJ, Okamoto I, et al (2013). Safety and efficacy of weekly nab ®-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-smallvcell lung cancer. Ann Oncol, 24(2):314-321. Tarceva® (erlotinib) [prescribing information] (2010). Melville, NY: OSI Pharmaceuticals, Inc. Temel JS, Greer JA, Admane S, et al (2011). Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 29(17):2319-2326. Temel JS, Greer JA, Muzikansky A, et al (2010). Early palliative care for patients with metastatic non-small cell lung cancer. N Engl J Med, 363(8):733-742. Vectibix® (panitumumab) prescribing information] (2013). Thousand Oaks, CA: Amgen, Inc. Yang JC, Schuler MH, Yamamoto N, et al (2012). LUX-Lung 3: A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. J Clin Oncol, 30(18 Suppl). Abstract LBA7500. Yang JC, Shih JY, Su WC, et al (2012). Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol, 13(5):539-548.