SPECTRAL SIMULATIONS
advertisement

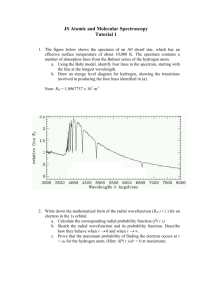
SPECTRAL SIMULATIONS Purpose: 1. To predict the transition of electrons from one energy position in an atom to another by examining their line spectra 2. To be able to identify an unknown element from its line spectra, simply by observing the electrons’ transitional states Procedure: Go to the following simulation: http://phys.educ.ksu.edu/vqm/html/emission.html 1. Put the hydrogen lamp into the power source. By hitting the “Add Energy Level” button, you add an energy level onto the eV scale. 2. The energy levels can be increased or decreased in their amount of energy by moving the mouse on the LEFT side of the scale. 3. The electron transition can be graphically shown by drawing arrows on the right side of the scale. 4. Try and figure out what transitions must take place in the hydrogen atom in order to produce the visible colors of light we know hydrogen produces by adjusting the energy levels. 5. Using E = RH (1/n2) as a guide, set the bottom line on your graph to an appropriate energy. Think about energy levels and electron transitions. You will have to calculate the energy of an electron at n=2 (HINT - An eV is an electron volt, which is equal to 1.6 x 10-19 Joules. It is defined as the amount of kinetic energy absorbed by an electron when it is hit with one volt of electricity). Try and have all of the other lines arrow down to this line! 6. What does each transition in hydrogen to produce visible light have in common? ___________________________________________________ 7. After finishing all of the transitions that must take place, print off a copy of the graphic and attach it to this worksheet. 8. What was the energy for an electron at n=2, in both Joules and eV, as calculated in number 5? 9. Bohr derived an equation for the drop of an electron from any one energy level to any other energy level in a hydrogen atom. Using Bohr’s equation, calculate the energy released when an electron drops from any one energy level to energy level two. Using the simulation, prove that your energy calculation is accurate (use the above hint as a guide!). 10. Choose any other gas tube, and show the electron transitions that must take place to produce visible light in that atom. Print off a copy of the graphic and attach it to this worksheet. 11. Explain why, even if your gas tube shares similar colors with hydrogen, why they might not have the same electron transitions.