Chapter 5, Section 3 Book Work Answers
advertisement

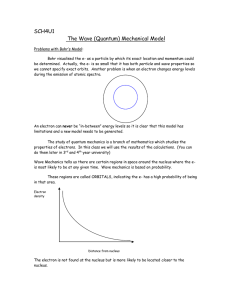
Honors Chemistry - Chapter 5, Section 3 Book Work Questions and Answers 16. How are wavelength and frequency related? a. Inversely proportional which means as one increases, the other decreases 17. Describe the cause of atomic emission spectrum of an element. a. Atoms absorb energy and electrons move to higher energy levels. When electrons fall back down to their ground states, they release energy in the form of light. Depending on the amount of energy released, the light will be different colors. 18. How is the change in electron energy related to the frequency of light emitted in atomic transitions? a. The light emitted by an electron moving to a lower energy level has a frequency directly proportional to the energy change of the electron. 19. How does quantum mechanics differ from classical mechanics? a. Classical mechanics describes the motion of large bodies. Quantum mechanics describes the motion of subatomic particles and atoms as waves. 20. The lines at the ultraviolet end of the hydrogen spectrum are known as the Lyman series. Which electron transitions within an atom are responsible for these lines? a. All electron transitions that end in the first energy level (on n=1) 21. Arrange the following in order of decreasing wavelength: a. infrared radiation from a heat lamp b. dental x-rays c. signal from a shortwave radio station a. Radio > Infrared > X-rays or C > A > B