levonorgestrel 1500mcg
advertisement

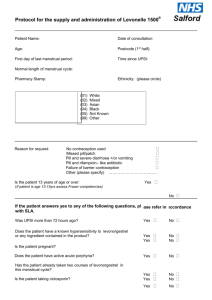
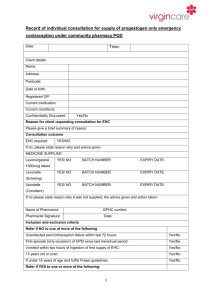
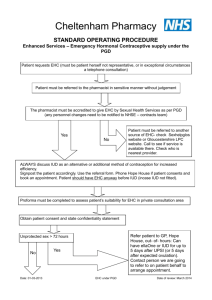
Draft National Template Patient Group Direction for the supply of: levonorgestrel 1500mcg by Community Pharmacists This direction was prepared as a template to assist organisations to prepare their own documents. It is the responsibility of local organisations to check the accuracy of the information, that the information is up to date, and to amend as required for local use. PGD EC Levonorgestrel v1 1 2 December 2014 Patient Group Direction for the Supply of: levonorgestrel 1500mcg Name and address of organisation: Date PGD comes into effect: Review date: (2 years or less) Expiry date: (2 years or less) Name of Medicine Approved name: levonorgestrel 1500mcg Professionals to whom PGD applies Community pharmacists Lead Doctor’s name, job title, signature and date: Lead Pharmacist’s name, job title, signature and date: Lead Nurse's name, job title, signature and date: On behalf of the Health Organisation name, job title, signature and date: PGD EC Levonorgestrel v1 2 2 December 2014 Clinical Condition Emergency contraception of unprotected sexual intercourse (UPSI) or failure of other contraceptive method; Note on mid-cycle use: The PGD emphasises the importance of additional referral for IUD for women presenting having had UPSI in mid cycle (see mid cycle calculator in appendix 1) Criteria for inclusion: Patient is aged 13 years or over And Presents in person at the community pharmacy within 72 hours of unprotected sexual intercourse (UPSI) or failure of a contraceptive method Or Presents in person at the community pharmacy between 72 and 96 hours following unprotected sexual intercourse (UPSI) or failure of a contraceptive method and where ulipristal is contraindicated and it is impracticable for the patient to be referred for insertion of an IUD. (Under 16 year olds are included provided they meet the criteria of the Fraser Guidelines on consent to medical treatment. The patient has capacity to understand the nature and purpose of the treatment and gives their consent to providing the relevant clinical information. In addition, if patient is under 13 years of age a referral to social services should be made in line with the Child Protection Procedures of the Health Board.) Remember a more effective alternative is a copper intra-uterine device (Cu-IUD). NB: If patient has vomited within 3 hours of taking a dose of levonorgestrel 1500mcg a further supply can be given provided patient is still within 96 hours of UPSI. Patient gives their consent to providing the PGD EC Levonorgestrel v1 3 2 December 2014 relevant clinical information to the pharmacist once pharmacist has assessed their capacity to understand the nature and purpose of the treatment Emergency contraception decision support guide for pharmacists (see appendix 2) BNF note: Levonorgestrel is effective if taken within 72 hours of unprotected intercourse and may also be used between 72 and 96 hours after unprotected intercourse (unlicensed use) Criteria for exclusion: Patients aged 12 years or under Patient who the pharmacist has assessed as not having capacity to understand the nature and purpose of treatment Patients who do not agree to share relevant clinical information or there is no valid consent Requests made by third parties on behalf of patient UPSI occurred more than 72 hours ago (refer to ulipristal acetate PGD) or UPSI occurred between 72 and 96 hours ago and ulipristal acetate is contra-indicated Patients who are/or think may be pregnant Patients whose last menstrual period (LMP) was more than 4 weeks ago (Note: some women have a longer cycle than 28 days so manage accordingly) Patients whose last LMP was abnormal/unusual Patients who have delivered a baby within last 3 weeks (EC not required in these circumstances) Patients taking ciclosporin Patients taking anticoagulants Patients with unexplained vaginal bleeding PGD EC Levonorgestrel v1 4 2 December 2014 Patients with severe hepatic dysfunction Patients with severe malabsorption syndrome e.g. porphyria Hypersensitivity to levonorgestrel or any of the tablet ingredients/excipients Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption are excluded from treatment with levonorgestrel under this PGD as this medicine may contain lactose monohydrate. Cautions (including any relevant action to be taken): Severe malabsorption syndromes e.g. severe diarrhoea, Crohn’s. The Faculty of Sexual and Reproductive Health (FSRH) advise that oral contraception may be less reliable in women with inflammatory bowel disease who have malabsorption due to severe small bowel disease or resection, or who have vomiting or severe diarrhoea for more than 24 hours. Refer to sexual health services for IUD/follow up Repeat use within a menstrual cycle is not advisable Action if excluded: All excluded patients should be referred to a Sexual Health Clinic or GP practice. Patient aged 12 years or under - the Child Protection Team must be contacted for children of 12 years and under, who present having had sexual intercourse. If unprotected sex was within the last 5 days (120 hours) the patient may be suitable for IUD (intrauterine device) insertion. Referral should be made in a suitable timeframe to allow this to happen. Only in circumstances where it is impracticable for the patient to be referred, should a supply of levonorgestrel be made more than 72 hours after UPSI. Seek further advice: PGD EC Levonorgestrel v1 To be completed by LHB according to local arrangements. Know the referral pathway into local sexual and reproductive health services 5 2 December 2014 or how to contact the local lead doctor for sexual and reproductive health for medical advice. Description of Treatment Name of Medicine Approved name: Levonorgestrel 1500mcg Proprietary name: Legal status: Prescription Only Medicine (POM) Form: Tablet Strength: Dosage: 1500mcg Take ONE tablet as a single oral dose as soon as possible after UPSI (preferably within 12 hours but no later than 72 hours unless as specified above). If the patient is using an enzyme inducing medication (refer to BNF chapter 7.3.5 Emergency Contraception for details) then TWO tablets should be taken as a single dose (total dose 3000mcg). This is an unlicensed indication for levonorgestrel. Oral 1500mcg as a single dose Route of Administration: Frequency of Administration: 3000mcg if patient using enzyme inducing medication NB: If patient has vomited within 3 hours of taking a dose of levonorgestrel the dose can be repeated. Total daily dose: 1500mcg Or 3000mcg (if patient taking enzyme inducing medication) Duration of treatment: Total treatment quantity: Use outside terms of summary of product characteristics: PGD EC Levonorgestrel v1 Single oral dose One or two 1500mcg tablets Levonorgestrel is not recommended in children and very limited data are available in women under 16 years of age. 6 2 December 2014 The use of two levonorgestrel 1500mcg tablets if the patient is taking enzyme-inducing drugs is an unlicensed indication. However this is recommended in the current BNF. Use between 72 and 96 hours after UPSI See Ulipristal acetate PGD. If ulipristal is contraindicated levonorgestrel may be considered for use between 72 and 96 hours after UPSI, but women should be informed of the limited evidence of efficacy and that such use falls outside the license of the product. If >72 hours but <96 hours after UPSI advise that the option of insertion of an IUD is the recommended option but that levonorgestrel is supported by evidence from a World Health Organisation trial. Use of levonorgestrel does NOT stop a woman from having the IUD fitted therefore women should be supplied levonorgestrel AND be referred for an IUD. Use more than once in a cycle Use more than once in a cycle is not advised because of the possibility of cycle disturbance. However it is supported by guidance from the Faculty of Sexual and Reproductive Healthcare. Explain to patient that advice differs from patient information leaflet and the reasons for this. Very common Adverse reactions: PGD EC Levonorgestrel v1 7 Bleeding not related to menses Delay of menses Headache Nausea Low abdominal pain Fatigue 2 December 2014 Common Breast tenderness Delay of menses >7 days Irregular bleeding and spotting Dizziness Diarrhoea Vomiting (This list is not exhaustive. Please refer to current SPC for further information.) Reporting procedure of adverse reactions: Side effects should be recorded in the patient record/notes. Refer to a GP where necessary. Consider reporting via the MHRA yellow card reporting scheme. Verbal advice for patient: Advice on likely side effects, action needed if vomiting occurs and delayed menses. All patients should be advised on the option of an emergency intra-uterine device (IUD) as an alternative. An IUD is more effective than hormonal emergency contraception especially when there are multiple risks or the patient is towards the end of the 72 hour time limit. Explain that: the next period may be early or late that a barrier method of contraception needs to be used until the next period the patient should contact their GP if any lower abdominal pain occurs (possibility of an ectopic pregnancy) if their next menstrual period is more than 5 days overdue, abnormal bleeding occurs or pregnancy is suspected, they should attend a sexual health clinic with a urine sample to confirm or exclude pregnancy. Highlight that levonorgestrel is used for emergency contraception only. Suggest where appropriate that patient makes an appointment with their GP or sexual health clinic to discuss their on-going contraceptive needs. Remind the patient that the use of PGD EC Levonorgestrel v1 8 2 December 2014 levonorgestrel does not provide ongoing contraceptive protection and that she must continue to use another method for the remainder of the cycle. Advise patient that they can be tested and treated for sexually transmitted infections if necessary and sign-post to locally available services. If the patient is breast feeding advise that levonorgestrel is not thought to be harmful. However potential exposure of the baby to levonorgestrel can be reduced if the mother takes immediately after feeding. Written information for patient: The following must be provided: A product Patient Information Leaflet (PIL) A leaflet on currently available forms of contraception Information on local sexual health services Follow Up: With sexual health service 3 weeks later for pregnancy testing and contraceptive/sexual health advice. Arrangements for referral for medical advice: Refer to a GP or sexual health clinic as necessary. Records of supply for audit: Maintain a record of the supply, batch number and expiry and any advice given (complete EHC community pharmacy proforma) The pharmacist must document Fraser Competence for clients under (or appearing to be under) 16 years of age. The pharmacist will be required to complete the National Electronic Claim and Audit Form (NECAF) for each consultation. NB: Records may need to be shared with LHB representatives subject to appropriate patient confidentiality protocols. Staff Professional qualifications: PGD EC Levonorgestrel v1 Community Pharmacists currently registered with the General Pharmaceutical Council 9 2 December 2014 (GPhC). Training: The Pharmacist must have completed the Wales Centre for Pharmacy Professional Education’s (WCPPE) All Wales procedure for accreditation to provide the Emergency Contraception Enhanced Service. http://www.wcppe.org.uk/assessment/enhance d-services/ehc-accreditation/emergencyhormonal-contraception The pharmacists must have a certificate demonstrating compliance with the National Competence and Training Framework for the service The pharmacist’s name must be included in the All Wales Pharmacy Database for the service. The pharmacist where required by the Local Health Board, has successfully completed an enhanced Criminal Records Bureau (CRB) check The pharmacist must handle all requests for emergency hormonal contraception in a sensitive and non-judgmental manner, and act in accordance with guidance issued by the General Pharmaceutical Council on consent, raising concerns and confidentiality. The pharmacist must be competent to assess a patient’s capacity to understand the nature and purpose of the treatment in order to give or refuse consent. Competency assessment: The pharmacist must satisfy the requirements of WCPPE. Continuing education: The pharmacist must undertake regular CPD and maintain own level of competence and knowledge in this clinical area to provide the service. The pharmacist must have access to, and be familiar with, the latest Summary of Product Characteristics for the specific levonorgestrel PGD EC Levonorgestrel v1 10 2 December 2014 product supplied and BNF. Special Considerations / Additional Information: Pharmacy premises must respect a patient’s right to confidentiality and safety and provide an acceptable level of privacy. References: British National Formulary 67 March 2014 Emergency Contraception Guidelines, Faculty of Sexual & Reproductive Health Clinical Effectiveness Unit (2012) http://www.fsrh.org (accessed 14 March 2014) Drug Interactions with Hormonal Contraception. Faculty of Sexual & Reproductive Healthcare Clinical Guidance (updated 2012) http://www.fsrh.org/pdfs/CEUguidancedr uginteractionshormonal.pdf (accessed 7 July 2014) GPhC Guidance – Consent 2012, Raising Concerns 2012 , and Confidentiality 2012 http://www.pharmacyregulation.org/stan dards/guidance (accessed 18 Mar 2014) NICE Good Practice Guidance on Patient Group Directions http://www.nice.org.uk/mpc/goodpractic eguidance/GPG2.jsp (accessed 17 March 2014) This Patient Group Direction is to be read, agreed and signed by all registered community pharmacists authorised to operate the PGD. One copy should be given to each named community pharmacist; the original signed copy should be kept by the nominated member of staff with responsibility for PGDs within the pharmacy. This will usually be the Superintendent Pharmacist or Responsible Pharmacist. I confirm that I have read and understood the content of this patient group direction and that I am willing and competent to work under it within my professional code of conduct. Name of Pharmacist: GPhC Registration Number: Pharmacy location: Signature: Date: Signed copy to be returned to: PGD EC Levonorgestrel v1 11 2 December 2014 PGD EC Levonorgestrel v1 12 2 December 2014 Appendix 1: Cycle length (in days) Calculating the mid cycle range for varying cycle lengths. 1 2 3 4 5 6 7 8 Day 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 26 28 30 32 Mid cycle is shown in dark blue. Women who present having had UPSI during the mid cycle period must be advised of the risk of EC failure supplied and also be referred for IUD insertion Note: Coils can be fitted up to 5 days post UPSI and also up to 5 days after the earliest possible ovulation, so UPSI on day 8/ 9/10/11 etc (of a 28 day cycle ) can still have a coil up to day 19 even if they have had repeated UPSI. (Bar chart could be used to help show this) Appendix 2 Emergency contraception decision guide for community pharmacists (based on FSRH review document http://www.fsrh.org/pdfs/FSRH_ECDecisionGuide.pdf)