Clinical Situation
advertisement

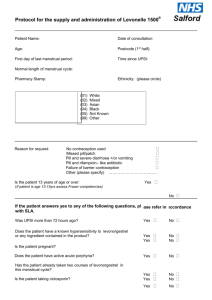
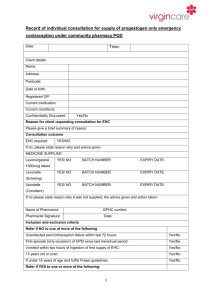
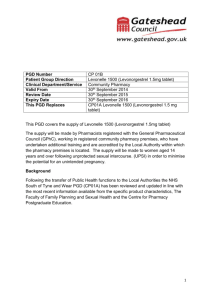
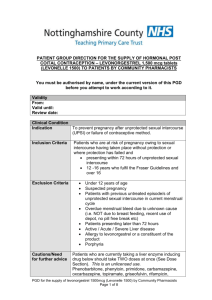
NATIONAL PATIENT GROUP DIRECTION TEMPLATE Purpose of this Document This document describes the content of any Patient Group Direction (PGD) used in conjunction with the All – Wales National Enhanced Service for Progestogen Only Emergency Contraception (POEC). Local Health Boards (LHB) are responsible for producing and authorising the PGD that it to be used for POEC supply from a pharmacy in their LHB area. The clinical content of the PGD operating in any LHB will be the same and is set out in this document. Pharmacists must read this document and sign an agreement to work within its terms. Pharmacists must also familiarise themselves with the format of the PGD available in each pharmacy at which they work. Management of the content of the National Patient Group Direction The content of the PGD shall be determined nationally. Variation to the content of the PGD can only be made with the agreement of the Welsh Assembly Government and only following consultation with Community Pharmacy Wales. Local Health Boards shall include the content of the national PGD, so far as it relates to the provision of the service, in their own template. Any pharmacist involved in the use of the PGD must read and agree to act only within the conditions specified there within. The pharmacist must sign an agreement to that effect and return this to the NHS Wales Shared Services Partnership. Clinical Situation Situation Patient presenting in person at the community pharmacy requesting emergency contraception for their own use. Inclusion Criteria Patient is aged 13 or over. Unprotected sexual intercourse (UPSI)/contraception failure within the last 72 hours. UPSI within the last 96 hours where, for reasons of unavailability of access to services within the 96 hour period following UPSI, it is impracticable for the patient to be referred for insertion of an Intra Uterine Device (IUD) or prescription of Ullipristal (see section entitled ‘Action if Excluded’). UPSI/contraception failure within the last 96 hours where patient has vomited within 3 hours of taking dose of levonorgestrel for emergency hormonal contraception. Patient gives their consent to providing the relevant clinical information to the pharmacist once pharmacist has assessed their capacity to understand the nature and purpose of the treatment Exclusion Criteria Longer than 96 hours since UPSI Patient is under 13 years. Patient who the pharmacist has assessed as not having capacity to understand the nature and purpose of treatment. Patient who does not give their consent to providing the relevant clinical information to the pharmacist. Patients who are/or may be pregnant. Patients who have delivered a baby within last 3 weeks (POEC not required in these circumstances). Patients taking ciclosporin. Patients with unexplained vaginal bleeding Patients with Severe hepatic dysfunction Patients with Severe malabsorption syndromes e.g. severe diarrhoea, Crohn’s disease Porphyria Hypersensitivity to levonorgestrel or any of the tablet ingredients/excipients (potato starch, maize starch, colloidal silica anhydrous, magnesium stearate, talc, lactose monohydrate). Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucosegalactose malabsorption as Levonelle® 1500 contains lactose. Requests made by third parties on behalf of the patient Action if Excluded All excluded patients should be referred to a Family Planning/Sexual Health Clinic or GP practice. Patient aged 12 years or under - the Child Protection Team must be contacted for children of 12 years and under, who present having had sexual intercourse If unprotected sex was within the last 120 hours the patient may be suitable for insertion of an IUD or prescription of Ullipristal. Referral should be made in a suitable timeframe to allow this to happen. Only in circumstances where it is impracticable for the patient to be referred, within the 120 hour period following UPSI, to a service providing IUD insertion or prescription of Ullipristal, should a supply of levonorgestrel be made more than 72 hours after UPSI. Characteristics of Persons and Premises Authorised Under the PGD Persons A pharmacist whose name is currently on register held by the General Pharmaceutical Council (GPhC) The pharmacist must have completed level 2 child protection training The pharmacists must have a certificate demonstrating compliance the National Competence and Training Framework for the service The pharmacist’s name must be included in the All Wales Pharmacy Database for the service. The pharmacist where required by the Local Health Board, has successfully completed an enhanced Criminal Records Bureau (CRB) check The pharmacist must undertake regular continuing professional development (CPD) and maintain own level of competence and knowledge in this clinical area to provide the service. Premises A premises specified in a Service Level Agreement (SLA) between the pharmacy contractor and the Local Health Board Description of Treatment Name of Medicine Levonorgestrel (Levonelle® 1500) Legal Status Prescription Only Medicine (PoM) Dosage Form/Strength Dose Tablet 1500microgram (mcg) Take ONE tablet (1500mcg) as a single oral dose as soon as possible after UPSI (preferably within 12 hours but no later than 72 hours unless as specified above). If the patient is using an enzyme inducing medication (refer to BNF Chapter 7.3.5 Emergency Contraception for details) then TWO tablets of levonorgestrel 1500 mcg should be taken as a single dose (total dose 3000mcg levonorgestrel). This is an unlicensed indication for levonorgestrel. Route of Administration Oral Frequency of Administration Single dose of 1500mcg Single dose of 3000mcg if the patient is using enzyme inducing medication If patient has vomited within 3 hours of taking a dose of levonorgestrel the dose can be repeated. Total Daily Dose 1500mcg or Duration of Treatment Total Treatment Quantity Use Outside 3000mcg (if patient is taking enzyme inducing medication) Single oral dose One or two 1500mcg tablet(s) Levonelle® 1500 is not recommended for women under Terms of Summary 16 years of age. of Product Characteristics The use of 2 Levonelle® 1500 tablets if the patient is (SPC) taking enzyme-inducing drugs is an unlicensed indication. However, this is the recommendation in the current BNF. Use between 72 and 96 hours after UPSI EHC may be considered for use between 72 and 96 hours after UPSI, but women should be informed of the limited evidence of efficacy and that such use falls outside the licence of the product. If >72 hours but <less 120 hours from UPSI, advise that the option of an insertion of an IUD is the recommended first –line treatment but that POEC is supported by evidence from a World Health Organisation trial. Use of POEC does NOT stop a woman from having the IUD fitted as well but may ensure some protection whilst more effective protection is sought. Use more than once in a cycle The product SPC advises against this because of the possibility of disturbance of the cycle. However, use more than once is supported by guidance from the Faculty of Sexual and Reproductive Healthcare. Adverse Reactions Explain to patient that advice differs from patient information leaflet and the reason for this. Very common Bleeding not related to menses, headache, nausea, low abdominal pain, fatigue Common Breast tenderness, delay of menses > 7 days, irregular bleeding and spotting, dizziness, diarrhoea, vomiting. Reporting Procedure for Adverse Reactions This list is not exhaustive. Please refer to the Summary of Product Characteristics (SPC) for further information. Side effects should be recorded in the patient medication record (PMR). Refer to a GP where necessary. Consider reporting via the MHRA yellow card reporting scheme Verbal Advice to Patients Advise on likely side effects, action needed if vomiting occurs, delayed menses and the failure rate. All patients should be advised on the option of an emergency IUD. Explain that: the next period may be early or late if the patient is taking an oral contraceptive or uses a contraceptive patch, but emergency contraception is indicated, that a barrier method of contraception may need to be used in addition to her usual method until she has taken the pill or applied the patch correctly for the appropriate time as advised in the most recent version of the British National Formulary. if the patient is not taking an oral contraceptive or using a contraceptive patch , a barrier method of contraception should be used until appropriate advice about ongoing contraception has been given the patient should contact their GP if any lower abdominal pain occurs (possibility of an ectopic pregnancy) if the patient has not had their period within 5 days of their expected date of menstruation, abnormal bleeding occurs or pregnancy is suspected, they should attend a Family Planning Clinic or GP with a urine sample to confirm or exclude pregnancy. highlight that levonorgestrel (Levonelle® 1500) is used for emergency contraception only. Suggest where appropriate that patient makes an appointment with their GP, Family Planning Clinic or Sexual Health Services to discuss their on-going contraceptive needs. Advise patient that they can be tested and treated for sexually transmitted infections if necessary and signpost the patient to locally available services. If the patient is breast-feeding advise that levonorgestrel (Levonelle® 1500) is not thought to be harmful. However potential exposure of the baby to levonorgestrel can be reduced if the mother takes the tablet immediately after feeding. Written information for Patient The following must be provided: A Levonelle® 1500 Patient Information Leaflet (PIL) A leaflet on currently available forms of contraception Information on family planning and sexual health services available within the Health Board area Follow Up None required Documentation The pharmacist must ensure maintenance of records of each supply including the date of supply, batch number and expiry date for each pack supplied. The pharmacist must document Fraser Competence for clients under (or appearing to be under) 16 years of age. The pharmacist will be required to complete the emergency hormonal contraception proforma for each consultation. The pharmacist will be required to complete the National Electronic Claim and Audit Form (NECAF) for each consultation. EMERGENCY CONTRACEPTION RECORD FORM This form is to be completed on each occasion the Emergency Contraception National Enhanced Service is provided. Pharmacy Stamp Completed forms should be retained for 10 years or until the client is 25 years age whichever is longer. Date of Consultation Patient Reference No Patient’s Age/Date of Birth Patient’s Postcode (complete as much as possible) Yes No Is the patient presenting in person? Refer If the patient is under 16 years of age, are they deemed Fraser competent? Refer Is the patient 13 years of age or over? Refer Was Last Menstrual Period (LMP) normal Refer Date of Last Menstrual Period (LMP) Has the patient had other episode(s) of UPSI in this cycle prior the episode for which she is presenting? Is the patient presenting more than 72 hours but less than 96 hours after UPSI Refer1 Refer1 Date and time of UPSI Has POEC been taken already in this cycle? Refer1 Does the patient have a known allergy to levonorgestrel or any other ingredient of Levonelle® 1500? Refer Yes Does the patient have severe hepatic dysfunction? Does the patient have any severe absorption difficulties e.g. severe vomiting or diarrhoea? Does the patient have any severe malabsorption syndrome e.g. Crohn’s disease? Does the patient have porphyria? Is the patient taking ciclosporin? Hours since UPSI Refer Refer Refer Refer1 Refer Has the patient delivered a baby within the last 3 weeks? Regular contraception Refer Refer Does the patient have unexplained vaginal bleeding? Is the patient taking enzyme inducing medication? (refer to BNF Chapter 7.3.5 Emergency Contraception for details) Reason for request Exclude (EHC not required) UPSI Condom Failure Patch COC Injection Other 0 – 24 Previous POEC use (months) No Other POP Implant IUD/S None 25 – 48 0–2 3-5 49 - 72 6+ Both oral and IUD emergency contraception options discussed 72 + Never Treatment Plan Levonelle® 1500 one tablet as single dose Levonelle® 1500 two tablets as single dose (enzyme inducers) No supply – patient presenting too late for treatment No supply – POEC not required 1. In these circumstances Pharmacists may use their professional judgement in determining whether to make a supply of levonorgestrel for an unlicensed indication Counselling Child protection issues considered/discussed Discuss mode of action Discuss failure rate Confirm next period my be early or late Discuss need for follow up including pregnancy test if next menstrual period is more than 5 days late of lighter than usual Discuss need to return if further UPSI Action to take if vomiting within 3 hours Discuss if appropriate unlicensed use and obtain consent Discuss need for future contraception Discuss risk of STIs Provide information on family planning and sexual health services available within the Health Board area Supervise dose on premises Details of Supply Batch Number Expiry Date Dose taken on the premises Declaration I have been counselled on the use of emergency hormonal contraception and understand the advice given to me by the pharmacist Patient Name Patient Address Patient Signature/Initials Date The action specified was based on the information given to me by the patient, which to the best of my knowledge, is correct. Pharmacist Signature Pharmacist GPhC Registration Number Date Fraser Guidance for Issuing Contraceptive Advice to those under 16 Fraser guidelines refer to the Department of Health guidance issued in 1986 on the provision of contraceptive advice and treatment to young people under 16 years of age (also formerly known as ‘Gillick Competence’). A doctor or any other professional would be justified in giving contraceptive advice and treatment to a young person under 16 without parental knowledge and consent provided if they were satisfied Yes No The young person, although under 16, understands the advice from the healthcare professional seen The young person cannot be persuaded to tell their parents they are seeking contraceptive advice The young person is likely to have intercourse with or without contraceptive treatment The young person’s physical or mental health is likely to suffer unless they receive contraceptive advice or treatment It is in the young person’s best interests to give contraceptive advice or treatment Declaration Pharmacist Signature Date Fraser guidelines for contraception Ref: Turner, J. (1985) “discretion to act in the interests of the girl – the Gillick Judgement. Journal of Medical Defence Union. Vol: Winter; 6-7. Fraser Guidelines