External review of application for the Expert
advertisement

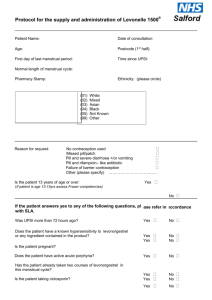
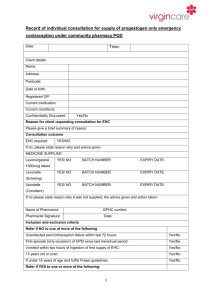
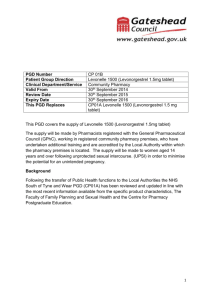
Summary of analysis of efficacy and safety of ethinylestradiol + levonorgestrel regimen and levonorgestrel only regimen in Emergency Contraception Definition: Emergency contraception is the use of a drug or device as an emergency measure to prevent pregnancy after unprotected intercourse. It is intended for occasional use and should not be used routinely. Emergency contraception could be useful in cases of sexual assault. Currently there are two dosage forms for emergency contraception on the WHO Essential Medicines List: ethinylestradiol + ٱlevonorgestrel tablet, 50 micrograms + 250 micrograms (pack of four) and, levonorgestrel tablets, 750 micrograms (pack of two) The application for deletion of the combination 4 tablet pack is supported by the high level of clinical evidence of its inferiority to the levonorgestrel only regimen (Cochrane Systematic Review)1. This meta-analysis of results of two trials comparing Yuzpe regimen (ethinylestradiol 50/100 micrograms and levonorgestrel 250/500 micrograms, 12 hours apart) and levonorgestrel (750 micrograms – two tablets taken with a 12 hour interval) with 2,633 women included in final calculations, confirmed that the levonorgestrel post-coital contraception was more effective and better tolerated than the Yuzpe regimen. The advantage in efficacy of levonorgestrel vs Yuzpe regimen was characterized by the Relative Risk of pregnancy (RR) of 0.51 with 95% Confidence Interval (CI): 0.31 to 0.84. In the later of the two trials2, the proportion of the prevented pregnancies (compared with the expected number without treatment) was 85% (74-93) with the levonorgestrel regimen and 57% (39-71) with the Yuzpe regimen (100/500 micrograms – higher doses). The better safety profile of levonorgestrel only regimen was confirmed by statistically and clinically significant fewer side effects with the resulting RR: 0.80, 95% CI: 0.74 to 0.86 (Cochrane Systematic review data)3. Nausea (16.1% vs 46.5%4 and 23.1% vs 50.5%5) and vomiting (2.7% vs 22.4%6 and 5,6% vs 18.8%7) occurred less frequently with levonorgestrel regimen (P<0.01). The latest WHO multicentre (15 family-planning clinics in 10 countries, 4,071 women participants) randomized trial of two regimens of levonorgestrel for emergency contraception (single-dose 1.5 mg and two-dose 0.75 mg 12 hours apart)8 demonstrated high and equal efficacy of both regimens if taken within five days of unprotected coitus. The pregnancy rates were 1.5% (20/1356) in women assigned single dose levonorgestrel and 1.8% (24/1356) in those assigned two-dose levonorgestrel (no statistical difference, p=0.83). The relative risk of pregnancy for single-dose levonorgestrel compared with twodose levonorgestrel was 0.83 with 95% CI 0.46-1.50. The results of this trial provided good evidence that a 1.5 mg single levonorgestrel dose can substitute two-dose regimen (0,75 mg 12 hours apart). The use of a single dose simplifies the use of levonorgestrel for emergency contraception without an increase in side effects. Conclusion: high quality clinical evidence has accumulated to form the most convincing argument to delete the ethinylestradiol + ٱlevonorgestrel tablet, 50 micrograms + 250 micrograms (pack of four) from the WHO Essential Medicines List and, add a new dosage form of levonorgestrel – 1.5 mg tablet. 1 Cheng L et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2002; 2: CD001324. 2 Task Force on Post-ovulatory Methods of Fertility Regulation. Randomized controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet 1998; 352:428-33 3 Cheng L et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2002; 2: CD001324). 4 Ho PC, Kwan MSW. A prospective randomized comparison of levonorgestrel with the Yuzpe regimen in post-coital contraception. Hum Reprod 1993; 8:389-9 5 Task Force on Post-ovulatory Methods of Fertility Regulation. Randomized controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet 1998; 352:428-33 6 Ho PC, Kwan MSW. A prospective randomized comparison of levonorgestrel with the Yuzpe regimen in post-coital contraception. Hum Reprod 1993; 8:389-9 7 Task Force on Post-ovulatory Methods of Fertility Regulation. Randomized controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet 1998; 352:428-33 88 Helena von Hertzen et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomized trial. Lancet 2002; 360: 1803