PGD Number CP 01B Patient Group Direction Levonelle 1500
advertisement

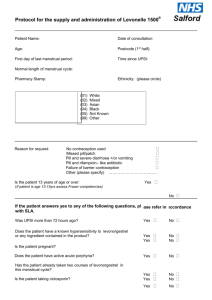
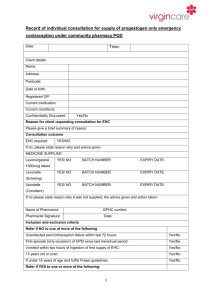
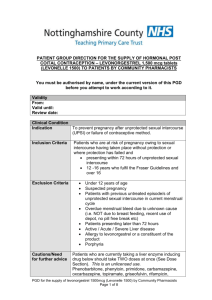
PGD Number Patient Group Direction Clinical Department/Service Valid From Review Date Expiry Date This PGD Replaces CP 01B Levonelle 1500 (Levonorgestrel 1.5mg tablet) Community Pharmacy 30th September 2014 30th September 2015 30th September 2016 CP01A Levonelle 1500 (Levonorgestrel 1.5 mg tablet) This PGD covers the supply of Levonelle 1500 (Levonorgestrel 1.5mg tablet) The supply will be made by Pharmacists registered with the General Pharmaceutical Council (GPhC), working in registered community pharmacy premises, who have undertaken additional training and are accredited by the Local Authority within which the pharmacy premises is located. The supply will be made to women aged 14 years and over following unprotected sexual intercourse. (UPSI) in order to minimise the potential for an unintended pregnancy. Background Following the transfer of Public Health functions to the Local Authorities the NHS South of Tyne and Wear PGD (CP01A) has been reviewed and updated in line with the most recent information available from the specific product characteristics, The Faculty of Family Planning and Sexual Health and the Centre for Pharmacy Postgraduate Education. 1 Title Head of Medicines Optimisation North of England Commissionin g Support Unit (NECS) (Pharmacist) Senior Medicines Optimisation Pharmacist Service Lead Service Lead Service Lead On behalf of Name (print) Gateshead, Janette South Stephenson Tyneside & Sunderland Local Authority Gateshead, South Tyneside & Sunderland Local Authority Gateshead Local Authority South Tyneside Local Authority Sunderland Local Authority Gateshead & South Tyneside Medical Practitioner (Lead Associate Specialist for Sexual Health Services) Medical Sunderland Practitioner Consultant Contraception Service Director of Gateshead Public Health Gateshead Director of South Public Health Tyneside South Tyneside Director of Sunderland Public Health Sunderland 1. Clinical Condition Signature Date Catherine McClelland Emma Gibson Janet Chandler Ben Seale Dr Janet Gallagher Dr Sarah Gatiss Carole Wood Amanda Healy Nonnie Crawford 2 Clinical Situation for which medicine is to be used. Criteria for inclusion Unprotected sexual intercourse or failure of a contraceptive method within 72 hours of presentation. Client having vomited within three hours of initial dose of Levonelle 1500, who decline the offer of an IUCD. Women aged 14 years and over presenting within 72 hours of UPSI or potential failure of a contraceptive method. Potential failure includes. Women who have severe diarrhoea and vomiting which may have reduced the efficacy of oral contraception. Women who have taken antibiotics which may have decreased the contraceptive effect of their regular contraceptive method and have not used an additional barrier method of contraception e.g. concomitant use of enzymeinducing rifamycins (such as rifabutin and rifampicin) and CHC (EHC is not required when using combined hormonal contraception (CHC) with antibiotics that are not enzyme inducers.) Noncompliance with dosage regime of oral contraceptives. i.e. delayed or missed pills. More than 90 days have elapsed since injection of Medroxyprogesterone. Having reason to believe that a barrier method has failed. Failed to use an additional barrier method of contraception when current methods have failed. Can confirm that their intrauterine contraceptive device (IUCD)/IUS or Subdermal implant has expired and has had a failure of contraception. Women who are taking oral contraception who have also taken prescribed, or OTC, medication that is known to interact with their oral contraception (producing a reduced effect). Women aged 14 years and over presenting within 72 hours of unprotected sexual intercourse who have taken one dose of Levonorgestrel and have vomited within three hours of taking the first dose of Levonorgestrel. (Levonelle 150 or Levonelle One Step). Criteria for exclusion. Women aged less than 16 years must be assessed and considered to be Fraser competent. UPSI more than 72 hours prior to presentation. Aged under 14 years 3 Aged under 16 years and not considered to be Fraser competent. Known hypersensitivity to Levonorgestrel or any excipients contained within the product. (Levonelle 1500). Suspected pregnancy. Prior use of Levonorgestrel within the same cycle. Other episode of unprotected sexual intercourse more than 72 hours ago. Unexplained or unusual vaginal bleeding. Young girls not having gone through menarche. Taking enzyme inducing drugs refer to current BNF for list. Taking drugs known to interact with Levonelle 1500 refer to current BNF for list. At risk of ectopic pregnancy Taking prescribed, or OTC, medication that is known to interact with Levonorgestrel producing toxicity (due to possible initiation of metabolism) Severe hepatic dysfunction Severe malabsorption syndrome Cautions/need for further advice If client prefers IUCD, after advice about efficacy, then refer to Contraception & Sexual Health service or GP. However, if appropriate, still offer Levonelle 1500 to client. Consider risks due to ovulation timing When there is suspected sexually transmitted disease (STI) refer to Sexual Health Services. Advice on Chlamydia screening service for under 25’s. Provide information on any appropriate local ‘condom supply schemes’ See supporting information file for specific information relating to services available locally. Action for excluded patients/patient declines Refer Contraception & Sexual Health service or GP. See supporting information file for specific information relating to services available locally. 2. Description of Treatment Name of medicine Dose Levonelle 1500 (Levonorgestrel 1.5mg tablet). Single tablet to be taken as soon as possible (provided dose is taken within 72 hours of unprotected sexual intercourse.) 4 Legal classification Route of administration Frequency of administration Period of administration Quantity to supply/administer Follow up Advice for patient Warnings/Adverse events POM Oral Single dose. Single dose (with possibility of second dose if vomiting occurs within 3 hours of first dose, providing that this second dose is still within the 72 hour window) One original pack containing one tablet. POM pack should be supplied. OTC pack should not be supplied. Refer to Contraception & Sexual Health service or GP if client experiences a missed or abnormal period. When there is suspected sexually transmitted disease (STI) refer to Sexual Health services. Advise on Chlamydia screening service for under 25’s. Provide information on any appropriate local “condom supply schemes”. Provide contraception leaflet Signpost to local contraceptive services. Inform client that if there is an abnormal period and/or abdominal pain then they should contact their local sexual health services or see their GP. See supporting information file for specific information relating to services available locally. Give patient information leaflet (PIL) and discuss as required. Reason treatment required. Mode of action. Failure rate and efficacy. What to do if vomiting occurs within 3 hours. (Advice may differ depending on timing of presentation with respect to 72 hour window) Then explain/discuss the potential side effects, and the likelihood of them occurring. Possible effects on menstrual cycle. What to do if period does not arrive/or is unusual. Effective contraception. Seeking medical advice. Treatment administration, Well tolerated, however, o Nausea and vomiting o Breast tenderness o Headache, Dizziness o Fatigue. Refer to SPC for additional details (a copy of the most recent SPC, at the time of development of the PGD is included in the supporting information pack. Be aware that this may have been superseded. Up to date SPCs can be accessed via the electronic Medicine 5 Arrangements for referral Records Compendium (emc), http:///www.medicines.org.uk/emc Client to report any suspected adverse effects believed to be associated with Levonelle 1500 to a Healthcare Professional or direct by using the Yellow Card system. Clients and Healthcare Professionals can log ADRs directly via the MHRA website (http://yellowcare.mhra.ove.uk/) Contraception & Sexual Health Service or GP See supporting information file for specific information relating to services available locally. Records should be kept that will demonstrate; Details of all drugs supplied for audit purposes. Fraser guidance fulfilled for under 16. Evidence of counselling and future contraception needs explored. Documentation of referral onward or advice sought. Records will be made on standard documentation. See supporting information pack for copies of documentation. 3. Staff Permitted to Supply/Administer under the PGD Qualifications required Additional requirements. Pharmacist registered with the General Pharmaceutical Council (GPhC) Pharmacist to provide evidence of completion of Centre for Pharmacy Postgraduate Education. Emergency contraception, e-learning and assessment Contraception open leaning programme and assessment. Safeguarding children and vulnerable adults, elearning and assessment. Pharmacists should attend CPPE Public Health workshop Emergency Contraception and associated pre- workshop task if accreditation to supply EHC under PGD is requested after 1st June 2014 or have attended a PCT training event on emergency hormonal contraception if initial accreditation to supply was requested prior to 1st June. Accreditation status will be reviewed annually. This should be in the form of a self-declaration of competency. The declaration will be made by submitting a signed form generated via the CPPE website. 6 (Declaration of qualifications and competence to deliver community pharmacy services – Emergency contraception). Each pharmacy must have a Standard Operating Procedure in place which covers the supply of Levonelle 1500 via this PGD. Education, Training Maintenance of knowledge, skills and competencies Continuing Professional by engaging in continuing professional development. Development Each practitioner is accountable for ensuring their skills and knowledge is kept up to date prior to supplying under PGD. AUTHORISATION OF NAMED HEALTH PROFESSINAL TO WORK WITHIN PATIENT GROUP DIRECTION. The direction must be read, agreed to and signed be each of the health professionals who wok within it. All professions must act within their appropriate Code of Professional Conduct. Pharmacy Contractors must ensure that the following staff are competent to provide care under this group direction including any training or education required. I have read and understood this Patient Group Direction and have received appropriate training and provided the Local Authority/Authorities within which I work with evidence of tis. I have been authorised to supply EHC under this PGD and I agree to work within its parameters. Date Name Designation Pharmacy Signature The above named professional(s) is (are) authorised to work within the parameters of the Patient Group Direction. Name Authorising Person: (Pharmacy Contractor / Manager / Director) …………………………………………………………………………………………… Title of Authorising Person:…………………………………………………………… Signature:……………………………………………………………………………….. Date:………/………../………… One copy retained by Local Authority. One copy retained by Contractor. One copy retained by Pharmacist 7