EHC Consultation Form (PDF Version)
advertisement

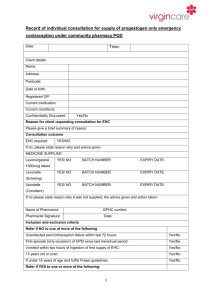
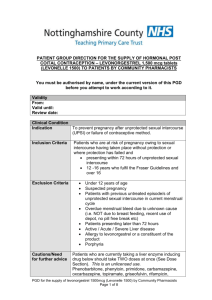
Protocol for the supply and administration of Levonelle 1500® Patient Name: Date of consultation: Age: Postcode (1st half): First day of last menstrual period: Time since UPSI: Normal length of menstrual cycle: Pharmacy Stamp: Ethinicity: (please circle) (01) (02) (03) (04) (05) (99) Reason for request: White Mixed Asian Black Not Known Other No contraception used Missed pill/patch Pill and severe diarrhoea +/or vomiting Pill and rifampicin– like antibiotic Failure of barrier contraception Other (please specify) ………………………………. Is the patient 13 years of age or over: Yes (If patient is age 13-15yrs assess Fraser competencies) No If the patient answers yes to any of the following questions, please refer in accordance with SLA. Was UPSI more than 72 hours ago? Does the patient have a known hypersensitivity to levonorgestrel or any ingredient contained in the product? Yes No Yes Yes No No Yes No Yes Yes No No Yes No Is the patient pregnant? Does the patient have active acute porphyria? Has the patient already taken two courses of levonorgestrel in this menstrual cycle? Is the patient taking ciclosporin? Is the patient less than 21 days post partum? (If yes EHC is not required) Yes No Is the patient 13-15 years old and not Fraser competent? Please retain on the premises for 3 years and 9 months Protocol for the supply and administration of Levonelle 1500® ® If the patient answers yes to any of the following questions, a supply of Levonelle 1500 can still be supplied but an IUD may be a more effective option: Has the patient had one previous episode of UPSI in the same cycle? Yes No Yes No Yes No Yes No (If yes and 3 weeks have passed offer a pregnancy test) Is the patient on anticoagulant medication? (If yes, advise patient to contact anticoagulant clinic after taking levonorgestrel) Is the patient taking an enzyme inducing drug e.g. carbamazepine, griseofulvin, oxcarbazine, phenytoin, primidone and other barbiturates, ritonavir, topiramate, nevirapine, rifabutin, rifampicin or St Johns Wort? (If yes offer two tablets as a single dose) Does the patient have breast cancer or a history of breast cancer, inflammatory bowel disease or a history of acute intermittent porphyria? Please ensure the following is discussed with the patient Mode of action discussed Failure rate discussed Side effects discussed Possible effects on foetus discussed Dose taken on premises? If dose not taken on premises, was a time for the dose negotiated? Follow-up discussed Future contraception discussed Discussed risk of ectopic pregnancy Discussed risk of S.T.I. with patient If patient under 25yrs, has a Chlamydia test been offered? Yes No Has a supply been made? If no, please state why …………………………………………………… Batch number of Levonelle 1500® supplied Expiry date of Levonelle 1500® supplied The above information is correct to the best of my knowledge. I have been counselled on the use of emergency contraception and understand the advice given to me by the pharmacist. Client’s signature: Date: The action specified was based on the information given to me by the client, which, to the best of my knowledge is correct. Pharmacist signature: Date: Pharmacist name (printed): Please retain on the premises for 3 years and 9 months