female clients for emergency hormonal contraception
advertisement

Patient Group Direction
For Supply Of Emergency
Hormonal Contraception By
Community Pharmacists
November 2005
City & Hackney TPCT PGD for EHC
Version printed: November 2005
Page 1 of 5
Patient Group Direction For Supply Of Emergency Hormonal
Contraception By Community Pharmacists
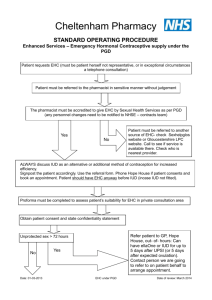
Rationale
To enable a pharmacist working in City and Hackney, who has received specific training and has
been assessed as competent, to supply emergency hormonal contraception in accordance with the
following patient group direction (PGD).
Professionals to whom this patient group direction may apply
Qualifications Required
Additional Requirements
Pharmacist (registered with RPSGB or PSNI)
Access to:
British National Formulary, latest edition
Levonelle 1500® summary of product characteristics
City and Hackney Teaching PCT medicines policies,
if appropriate
Information about services available to young people
Continuing Training
Requirements
It is the responsibility of the individual pharmacist to
ensure that they and their staff are competent in all aspects
of supply and administration of emergency hormonal
contraception and are updated on current medicines
policies.
THIS PATIENT GROUP DIRECTION WILL BE SUBJECT TO REGULAR REVIEW IN
LINE WITH CURRENT CLINICAL PRACTICE AND AT TIMES OF MAJOR CHANGE
Date of overall review of this document – November 2007
City & Hackney TPCT PGD for EHC
Version printed: November 2005
Page 2 of 5
Patient Group Direction for the
supply/administration of
to
LEVONORGESTREL 1500 MCG TABLETS
FEMALE CLIENTS FOR EMERGENCY HORMONAL CONTRACEPTION
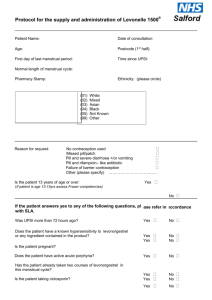
1. Clinical Condition
Define situation/condition
Criteria for inclusion
Prevention of pregnancy from unprotected sexual intercourse (UPSI)
in women under 26 years of age*.
UPSI defined as follows:
Penetration with or without ejaculation, or ejaculation on external
genitals and no contraceptive method used
Barrier method failure
Missed pills without alternative methods used
More than 89 days have elapsed since the last
medroxyprogesterone injection
More than 21 days post-partum if not breastfeeding
More than 42 days post-partum if fully breastfeeding
Potential Intra Uterine Contraceptive Device (IUCD) failure, e.g.
lost threads
*Treatment may be given to women over 26 in exceptional
circumstances, e.g. at weekends, after normal GP practice opening
hours
Women presenting within 72 hours of UPSI as defined above, or
women who have:
Severe diarrhoea and vomiting which may have reduced oral
contraceptive efficacy
Also been taking broad-spectrum antibiotic with a combined oral
contraceptive pill and have failed to use additional barrier method
during the course of the treatment
Received levonorgestrel emergency contraception (e.g. from GP,
purchased over-the-counter, PUCC), but have vomited within 3
hours of taking the dose (provided it is still within 72 hours of
UPSI)
Fraser Ruling: the Fraser ruling concluded that there is no lower age
limit for young women to understand sexual health issues. For clients
known/thought to be under 16 years of age, discussion with the young
person should explore their understanding of the advice given, and
they should be encouraged to involve their parents. The following
should also be considered:
Adverse effect on the physical or mental health of the young
person if advice or treatment withheld
Supply is in the best interests of the young person
The young person is likely to continue with sexual activity
without advice or treatment
Criteria for exclusion
City & Hackney TPCT PGD for EHC
Version printed: November 2005
Child protection: an assessment of risk of harm should be made for
clients under 16, including age of partner. Contact appropriate
authorities if there are any concerns – refer to the information
provided.
UPSI more than 72 hours ago
Known hypersensitivity to levonorgestrel or any ingredient
Page 3 of 5
Action if excluded
Action if patient declines
2. Description of treatment
Name of Medicine
POM/P/GSL
Dose/s
Route Method
Frequency
Total dose number
Follow up
Advice
Records
City & Hackney TPCT PGD for EHC
Version printed: November 2005
contained in the product
Suspected pregnancy
Unexplained or unusual vaginal bleeding
Acute severe liver disease
Active acute porphyria
Severe intestinal malabsorption syndromes, e.g. Crohn’s Disease
Clients taking enzyme-inducing drugs
If more than 72 hours since episode of UPSI, a post-coital IUCD
is an alternative option
Refer to family planning clinic or GP for advice about regular
contraception
Document refusal/action taken in client’s record.
Levonorgestrel 1500 microgram tablets (Levonelle 1500®)
POM
One tablet to be taken as soon as possible, preferably within 12 hours
and no later than 72 hours of UPSI
Oral
Single episode of treatment
1500 microgram tablet x 1
Clients should be provided with information and advice about
regular contraception – an information pack should be provided to
all clients, whether included or excluded
Clients should be given 3 condoms
Client may require follow-up by GP or family planning clinic e.g.
for missed or abnormal period
If a sexually transmitted infection is suspected, refer to genitourinary medicine clinic
Explain treatment and administration including advice if vomiting
occurs
Discuss side-effects and administration with the client and
provide a manufacturer’s patient information leaflet
Advise client that she could still become pregnant. If menstrual
periods are delayed by more than 5 days or are abnormal in any
way (light, heavy or painful), client should seek medical advice
If a pregnancy has occurred, following failure of levonorgestrel
treament, the client should contact GP/Family planning clinic for
follow-up to ensure that it is not ectopic
Seek medical advice if there is any lower abdominal pain
Stress need to use a reliable barrier method, e.g. condom,
diaphragm or cap, until the next menstrual period, or abstain from
sexual intercourse
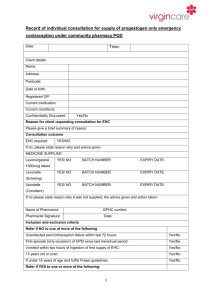
Client’s name or initials, date of birth and GP details, if they are
willing to share these details. The first part of the post-code and
the number from the 2nd part should be recorded for analytical
purposes
Reason for inclusion
Advice given to patient
Batch number and expiry date
Name of pharmacist who supplied the medication
Details of any adverse drug reaction and actions taken including
documentation in the client’s medical record via GP
Page 4 of 5
Patient Group Direction For Supply Of Emergency Hormonal Contraception By
Community Pharmacists
DECLARATION
DECLARATION by City & Hackney Teaching Primary Care Trust:
This PGD has been authorised by:
Enquiries relating to this PGD should be addressed to:
Head of Pharmacy & Prescribing, City & Hackney tPCT, St Leonard’s, Nuttall Street, London N1 5LZ.
Tel: 020 7683 4454
Fax: 020 7683 4464
Date of review: November 2007
This PGD is available on the tPCT intranet
{click on ‘Departments’, then click on ‘Primary Care Dev.’, then click on ‘Prescribing’, then click
on ‘Patient Group Directions’}
I have been appropriately trained to understand the criteria listed and the administration
required to supply emergency hormonal contraception in accordance with this Patient Group
Direction. I confirm that I am competent to undertake administration of these medicines.
Pharmacist Name:…………………………….
Pharmacy stamp:
RPSGB/PSNI registration number:
Signature:…………………..
City & Hackney TPCT PGD for EHC
Version printed: November 2005
Date:……………………….
Page 5 of 5