SS Acylation
advertisement

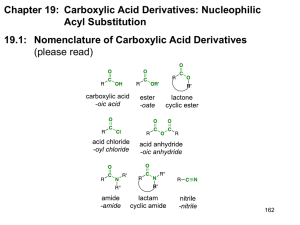
Acylation Acid Chlorides and Acid Anhydrides An ACYL group is the CARBONYL group with an ALKYL group attached so that the spare bond to attach to other molecules is from the carbonyl carbon. Like this:bond to other molecules The introduction of the group into another molecule is called ACYLATION. The reaction is used in the pharmaceutical (aspirin) and the textile (cellulose acetate or rayon) industries. Carboxylic acids contain the ACYL group but the OH group bonded to the ACYL group is a very poor leaving group and so carboxylic acids are not used as ACYLATING agents. Instead, two carboxylic acid derivatives are used. They are ACID CHLORIDES (or ACYL CHLORIDES) and ACID ANHYDRIDES. Of the two, ACID ANHYDRIDES are the preferred choice because: they are cheaper to manufacture; they are less reactive so the ACYLATING reactions are less dangerous and easier to control; ACYL CHLORIDES produce HCl as a product of ACYLATION, and this is a corrosive molecule, whereas ACID ANHYDRIDES produce a molecule of WATER. water easily hydrolyses ACYL CHLORIDES as is seen in the next section. ACID ANHYDRIDES are much less susceptible to hydrolysis. The main disadvantage of using ACID ANHYDRIDES is that the bi-product is either ETHANOIC ACID (for the reactions with WATER and ALCOHOLS) or the AMMONIUM or AMINE salts of ETHANOIC ACID (for the reactions with AMMONIA and AMINES). These bi-products are not very easy to remove, whereas the other reaction product with ACID CHLORIDES is HCl, which is volatile and easily separated. ACYL CHLORIDES or ACID CHLORIDES Acid (or acyl) chlorides have the structure: - The electron withdrawing effect of the carbonyl oxygen atom, coupled with that of the chlorine atom, make the carbonyl carbon atom quite positively charged and susceptible to nucleophilic attack by water (hydrolysis) to give the acid; by alcohols to give the ester; by ammonia to give the acid amide; by amines to give substituted amides. This is especially so because, in addition, the Cl atom is a good leaving group. They are made by the action of SULPHUR DICHLORIDE OXIDE (thionyl chloride) SOCl2 on CARBOXYLIC ACIDS. CH3COOH + SOCl2 CH3COCl + SO2 + HCl 1 ACID ANHYDRIDES The structure of ACID ANHYDRIDES is shown on the right. They are called ANHYDRIDES of ACIDS because if the atoms of one molecule of water are added to a molecule of an ACID ANHYDRIDE, two molecules of the CARBOXYLIC ACID are formed. They are not made by dehydrating carboxylic acids, but rather by ACYLATING their SODIUM SALTS. The ‘Na+’ ion of the sodium salt is replaced by the ACYL group. The equations and mechanisms follow. The mechanisms for the ACYLATION reactions using ACID ANHYDRIDES are not necessary for the course, only mechanisms using ACID CHLORIDES are required. Equations will be required for BOTH acid chlorides AND anhydrides. 1. Water CH3COCl + H2O CH3COOH + HCl The reaction is essentially a NUCLEOPHILIC ADDITIONELIMINATON reaction. 1. The lone pair from a water oxygen attacks the + carbon atom and the CARBONYL (C=O) double bond breaks. This is the ADDITION part of the reaction. 2 2. The C–Cl bond breaks and the Cl atom leaves. Simultaneously, the C=O double bond reforms. This is the ELIMINATION part of the reaction. 3. A proton leaves to produce the final neutral molecule. The NUCLEOPHILE (water, in this case) has undergone a NUCLEOPHILIC SUBSTITUTION. A HYDROGEN has been replaced by an ACYL group. Water is said to have been ACYLATED. The NUCLEOPHILE (water, in this case) has undergone a NUCLEOPHILIC SUBSTITUTION. A HYDROGEN has been replaced by an ACYL group. Water is said to have been ACYLATED. (repeated because it is important that the meaning of “ACYLATION” is understood. What has been “ACYLATED”? WATER. The ACYL group has substituted a HYDROGEN atom in water) The corresponding ACID ANHYDRIDE EQUATION:- (CH3CO)2O + H2O CH3COOH + CH3COOH The “left-over” product from the ANHYDRIDE. The product from ACYLATING water. 2. Alcohols CH3COCl + CH3OH CH3COOCH3 + HCl In industry, ETHANOIC ANHYDRIDE is used instead of ETHANOYL CHLORIDE for the reasons previously outlined. The equation is:- (CH3CO)2O ethanoic anhydride + CH3OH CH3COOCH3 methanol methyl ethanoate + CH3COOH ethanoic acid The carboxylic acid can be removed by the addition of SODIUM CARBONATE which neutralises it and converts it to the SODIUM SALT which is not volatile. The ESTER can be distilled off. This method is a much more satisfactory method of producing ESTERS than the CARBOXYLIC ACID plus ALCOHOL method because unlike the acid plus alcohol method, the ACYLATION method is rapid, not reversible, does not require a catalyst and produces a very good yield. ASPIRIN It has been known for some 2 400 years that chewing the leaves or the bark of Willow trees was found to relieve pain and suppress the symptoms of mild fever. In the mid eighteen hundreds, it was shown that the active ingredient responsible contained in the leaves was SALICYLIC ACID or 2-HYDROXYBENZOIC ACID. (right) A German scientist, KOLBE, discovered a very easy and cheap method of manufacturing SALICYLIC ACID. His method involved heating PHENOL (structure shown here on the left) with CARBON DIOXIDE under pressure. Unfortunately, the drug was 3 found to have some unpleasant side effects, such as irritation of the mouth, throat and stomach. Hofmann, another German scientist, investigated many derivatives of SALICYLIC ACID to find one that relieved pain in a similar way to SALICYLIC ACID, but without the unacceptable side effects. Eventually, ACYLATION of the PHENOLIC group (the OH) provided the answer. Here, the ‘H’ of the OH group is replaced by the ACYL group, CH3CO The systematic name for ASPIRIN is 2-ethanoyloxybenzenecarboxylic acid. Ethanoic Anhydride is used instead of ethanoyl chloride and the corresponding equation is shown below. 3. Ammonia CH3COCl + 2 NH3 CH3CONH2 + NH4 Cl An amide ETHANAMIDE A second molecule of ammonia is involved in the last stage. Being basic it can remove the proton. The equivalent reaction involving the ACID ANHYDRIDE is:- (CH3CO)2O + 2 NH3 CH3CONH2 + CH3COO NH4 ammonium ethanoate 4 When ALKYL groups replace ‘H’ atoms in AMMONIA, the new amine molecule becomes even more susceptible to attack by + carbon atoms. Expressed another way, the amine molecule is a more powerful nucleophile than the ammonia molecule it was made from and this leads to further ‘H’ atoms being replaced by alkyl groups. This is because the replacement of an ‘H’ in ammonia by an alkyl group makes the lone pair of electrons on the ‘N’ atom more available for nucleophilic attack because of the +I effect of alkyl groups. (see Reactions of Haloalkanes with ammonia). The reaction mechanism is reproduced here for convenience. This is followed by a further reaction where a molecule of chloromethane is attacked by the newly formed amine in preference to being attacked by ammonia because the amine is a stronger nucleophile. The +I inductive effect of the alkyl group makes the lone pair of electrons on the nitrogen atom more available for nucleophilic attack. In the reaction between ammonia and ethanoyl chloride where ETHANAMIDE is produced, the reaction stops at this stage and further substitution of the ‘H’ atoms in the NH2 group does NOT occur because the electron WITHDRAWING effect, or I, of the carbonyl group makes the lone pair of electrons on the ‘N’ atom LESS available for further nucleophilic attack. 4. Amines aminomethane primary Nmethylaminomethane secondary N,Ndimethylaminomethane tetramethylammonium ion tertiary quaternary 5 CH3COCl (CH3CO)2O + 2 C6H5NH2 CH3CONHC6H5 + C6H5NH3+ Cl phenylamine N-phenylethanamide phenylammonium chloride + 2 C6H5NH2 CH3CONHC6H5 + CH3COO [NH3 C6H5]+ phenylammonium ethanoate The Mechanism The proton is removed by another molecule of the amine, which, being BASIC, can remove the ACIDIC proton. The reaction stops at this stage, unlike in the case of reactions with HALOALKANES, which can form TERTIARY and even QUATERNARY AMINES, as described above. 6