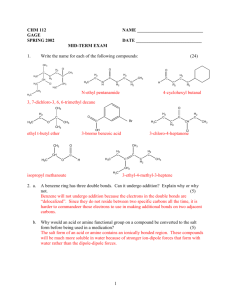
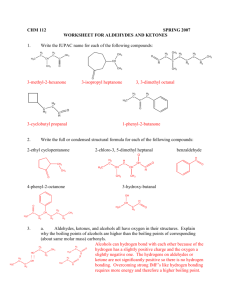
Aldehydes and ketones both contain a carbonyl group
advertisement

ACYLATION ACYL CHLORIDES & ACID ANHYDRIDES Acyl chlorides, for example ethanoyl chloride CH3COCl, contain the functional group C=O Cl Acid anhydrides, for example ethanoic anhydride (CH3CO)2O, contain the functional group C=O O C=O Both acyl chlorides and acid anhydrides are used to carry out acylation reactions. ACYLATION is the introduction of an RC=O group Acylation When acyl chlorides and acid anhydrides react with nucleophiles such as water, alcohols, ammonia or primary amines, they react via a nucleophilic addition – elimination reaction. Acyl chlorides and acid anhydrides both contain a carbonyl group. The carbonoxygen bond in the carbonyl group is polar, because oxygen is more electronegative than carbon. However, the presence of an additional electron-withdrawing group polarises the carbonyl group to a greater extent than in aldehydes and ketones. The carbon atom forms the positive end of the dipole. + - C=O Cl The increased electron-deficiency of the carbon atom makes acyl chlorides and acid anhydrides much more susceptible to attack by nucleophiles than aldehydes and ketones. Since the carbon atom of the carbonyl group is sp 2 hybridised, the first step in the mechanism is addition. Cl- ions, formed from acyl chlorides, and R.CO.O- ions, formed from acid anhydrides, are both good leaving groups; this means that elimination follows as a second step in the mechanism. The reactions taking place with water and alcohols are: ethanoyl chloride + water: CH3COCl + H2O CH3COOH + HCl carboxylic acid ethanoic acid ethanoyl chloride + ethanol: CH3COCl + C2H5OH CH3COOC2H5 + HCl ester ethyl ethanoate TOPIC 13.6: ACYLATION 1 ethanoic anhydride + water: (CH3CO)2O + H2O 2CH3COOH ethanoic acid ethanoic anhydride + ethanol: (CH3CO)2O + C2H5OH CH3COOC2H5 + CH3COOH ethyl ethanoate The mechanisms with water and alcohols are identical. H3C C Cl O nucleophilic addition CH3 Cl C H3C .. elimination O C +O H :O H +O H H O H H :Cl - H3C C O HCl HO ethanoic acid H3C C Cl O nucleophilic addition :O R H You need to know the mechanisms of the reactions with acyl chlorides. The mechanisms of the reactions with acid anhydrides are not required. CH3 Cl C H3C .. O - elimination C +O R +O H O R H :Cl - H3C C RO O HCl alkyl ethanoate The mechanisms with ammonia and primary amines are identical. Here, an intermediate cation is formed, from which a proton is removed by a second molecule of ammonia or primary amine, acting as a base. TOPIC 13.6: ACYLATION 2 The reactions taking place with ammonia and amines are: ethanoyl chloride + ammonia: CH3COCl + 2NH3 CH3CONH2 + NH4Cl primary amide ethanamide ethanoyl chloride + methylamine: CH3COCl + 2CH3NH2 CH3CONHCH3 + CH3NH3Cl secondary N-methyl ethanamide amide ethanoic anhydride + ammonia: (CH3CO)2O + 2NH3 CH3CONH2 + CH3COONH4 ethanamide ethanoic anhydride + methylamine: (CH3CO)2O + 2CH3NH2 CH3CONHCH3 + CH3COONH3CH3 N-methyl ethanamide The mechanisms with ammonia and primary amines are identical. H3C C Cl O nucleophilic addition CH3 Cl H3C .. O - elimination C C + H N H .. H N H H H O N+ H H H :NH3 :Cl H3C C O NH4Cl H2N ethanamide H3C C Cl O nucleophilic addition .. H N R CH3 Cl C + H N R H3C .. elimination OC H H N+ R H H :NH2R :Cl H3C C O RHN N-alkyl ethanamide TOPIC 13.6: ACYLATION 3 O RNH3Cl Acylation Reactions Large scale acylation reactions are usually carried out with acid anhydrides. Ethanoic anhydride is manufactured on a large scale as an acylating agent and is preferred to ethanoyl chloride because: it is cheaper than the acyl chloride it is less corrosive, since HCl is not produced it is less easily hydrolysed by atmospheric moisture it is less dangerous to use: it is less reactive than the acyl chloride and its reactions are therefore less violent Ethanoic anhydride is used in the manufacture of the man-made fibre, Tricel from cellulose and in the manufacture of aspirin (2-ethanoyloxybenzenecarboxylic acid), a widely used analgesic drug. Aspirin is made by the acylation of 2-hydroxybenzenecarboxylic acid. COOH COOH OH + (CH3CO)2O TOPIC 13.6: ACYLATION 4 OCOCH3 + CH3COOH