Chem 110b–Section 2
advertisement

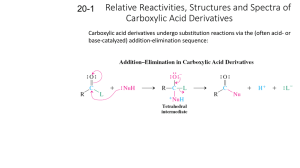
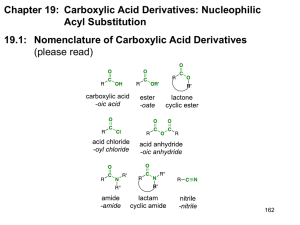
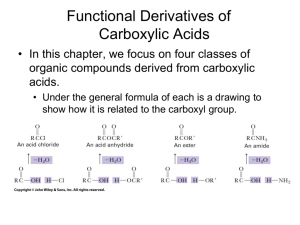
Chem 110b–Section 2 Exam 3 Review Sheet Chapter 18 Properties of carboxylic acids (COOH): nucleophilic site=carbonyl carbon, inductive and charge distribution substituents effects on acidity, often found as hydrogen bonded dimers. Nomenclature of COOH, acyl chlorides, anhydrides, esters, and amides. Preparation of COOH: oxidation of CHO or 1° ROH with H2CrO4, benzylic oxidation (KMnO4/NaOH) gives benzoic acid derivatives, haloform reaction, oxidative cleavage of alkenes (either KMnO4/NaOH or O3 then H2O2), carbonylation of organolithium or Grignard reagents with CO2 (dry ice). Preparation of derivatives from COOH including mechanisms: acyl chlorides (SOCl 2), anhydrides (acyl chloride+COOH+pyridine), esters via Fisher esterification (H2SO4/H2O/heat), amides via dehydration (high temperatures) or activated coupling (using DCC-excellent for making “peptide” bonds between amino acids). Relative reactivity of all derivatives: acyl chlorides > anhydrides > esters > amides. Use resonance to explain the order of reactivity and observed difference in the frequency for each type of carbonyl in the IR spectrum. Remember that IR should not be used alone to determine what type of carbonyl is present (CHO and ketones=1710-1750 cm-1). Any COOH derivative can be prepared from a more reactive derivative, but NOT from a less reactive one. Conditions, mechanisms and products: acyl chlorides to anhydrides, esters, and amides, anhydrides to esters and amides, ester to amides, Baeyer-Villager oxidation of ketones to esters, reduction of esters/acyl chlorides to CHO, reduction of esters to 1° ROH (no mechanism), addition of R–M reagents to esters to give 3° ROH, acid catalyzed and base promoted (saponification) ester hydrolysis, acid catalyzed and base promoted amide hydrolysis, amide dehydration to nitriles (no mechanism), hydrolysis of nitriles under acid or base conditions. Lactones/lactams in antibiotics, esters as “protecting groups” for COOH, saponification in the production of soap, relative rates of ester versus amide bond hydrolysis. Questions you should be able to answer: 1. Why are COOH always protonated on the carbonyl rather than hydroxyl oxygen? 2. Where do the oxygens, present in an ester after Fisher esterification, originate? 3. Why do amide carbonyls show up around 1680-1700 cm-1 and anhydrides around 1740-1790 cm-1? You should invoke both an equation and resonance to answer this question. 4. Why is only one equivalent of nucleophile added to a cyclic anhydride? 5. How do soaps remove dirt? Why does this work? 6. When is an amide not an amide? Hammett Equation Where do K, Ko, k, ko, , and come from? Which can be calculated? Which can be looked up? What is the standard reaction that all others are compared to? What does it mean if is positive? What does it mean if is negative? Predict the sign of for a given reaction by considering the species generated during the rate-determining step. The trend in substituent values is (+) = electron withdrawing and (–) = electron donating.