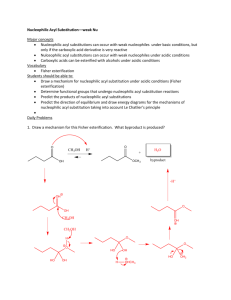
Chapter 12: Acyl substitution reactions
advertisement

Organic Chemistry With a Biological Emphasis: An Option for Second-Semester Organic Chemistry at the University of Minnesota, Morris Tim Soderberg HHMI/AAMC SFFP Report Competency E4, part 6: Demonstrate knowledge of the chemistry of carbon-containing compounds relevant to their behavior in an aqueous environment. Examples: • Recognize major types of functional groups and chemical reactions. • Explain how molecular structure and geometry, including chirality, relate to chemical reactivity. • Explain the chemical principles that allow structural inference about bioorganic molecules based on common spectroscopic analyses, such as NMR, UV/visible/IR absorption, or X-ray diffraction. • Apply knowledge of the chemistry of covalent carbon compounds to explain biochemical reactions. HHMI/AAMC SFFP Report Competency E4, part 6: Demonstrate knowledge of the chemistry of carbon-containing compounds relevant to their behavior in an aqueous environment. Examples: • Recognize major types of functional groups and chemical reactions. • Explain how molecular structure and geometry, including chirality, relate to chemical reactivity. • Explain the chemical principles that allow structural inference about bioorganic molecules based on common spectroscopic analyses, such as NMR, UV/visible/IR absorption, or X-ray diffraction. • Apply knowledge of the chemistry of covalent carbon compounds to explain biochemical reactions. MR5 Committee Recommendations Chemical and Physical Foundations of Biological Systems (second of four test sections) . . . Understanding the mechanical, physical, and biochemical functions of human tissues, organs, and organ systems is important to the study of medicine. This section will test the extent to which examinees know the basic chemical and physical principles that underlie the mechanisms operating in the human body. It will further test examinees’ ability apply their understanding of these general principles to living systems. MR5 Committee Recommendations Chemical and Physical Foundations of Biological Systems (second of four test sections) . . . Understanding the mechanical, physical, and biochemical functions of human tissues, organs, and organ systems is important to the study of medicine. This section will test the extent to which examinees know the basic chemical and physical principles that underlie the mechanisms operating in the human body. It will further test examinees’ ability apply their understanding of these general principles to living systems. The Preview Guide to MCAT2015 Foundational Concept 5: The principles that govern chemical interactions and reactions form the basis for a broader understanding of the molecular dynamics of living systems. The content categories for this foundational concept include: 5A. Unique nature of water and its solutions 5B. Nature of molecules and intermolecular interactions 5C. Separation and purification methods 5D. Structure, function, and reactivity of biologically-relevant molecules 5E. Principles of chemical thermodynamics and kinetics The Preview Guide to MCAT2015 Foundational Concept 5: The principles that govern chemical interactions and reactions form the basis for a broader understanding of the molecular dynamics of living systems. The content categories for this foundational concept include: 5A. Unique nature of water and its solutions 5B. Nature of molecules and intermolecular interactions 5C. Separation and purification methods 5D. Structure, function, and reactivity of biologically-relevant molecules 5E. Principles of chemical thermodynamics and kinetics “Leaders in science education say that some of the most important foundational concepts in the sciences ask students to bring together information from different disciplines.” (The Preview Guide for MCAT2015, p. 8) Organic textbooks place a heavy emphasis on lab synthesis – there is a need for a more biology-focused option. Current solution at UMM: First semester: Two sections, both covering same chapters of Bruice text Second semester: two options a 1: ‘Standard’ o-chem course, continues with Bruice 2: Bio-flavored course, switches to ‘Organic Chemistry With Biological Emphasis’. Cell Biology prerequisite Open access textbook: online (Chemwiki at UC-Davis) pdf, print (lulu.com) Mechanistic organization, biological/medical examples take center stage Extensive references/links to primary literature NOT a Biochemistry text Contents of ‘Organic Chemistry With a Biological Emphasis’ vol II Chapter 10: Phosphoryl transfer reactions Chapter 11: Nucleophilic carbonyl addition reactions Chapter 12: Acyl substitution reactions Chapters 13/14: Reactions at the a-carbon Chapter 15: Electrophilic reactions Chapter 16: Oxidation and reduction reactions Chapter 17: Radical reactions (Bio-flavored course at UMM covers chapters 10-17 plus NMR) Contents of ‘Organic Chemistry With a Biological Emphasis’ vol II Chapter 10: Phosphoryl transfer reactions Chapter 11: Nucleophilic carbonyl addition reactions Chapter 12: Acyl substitution reactions Chapters 13/14: Reactions at the a-carbon Chapter 15: Electrophilic reactions Chapter 16: Oxidation and reduction reactions Chapter 17: Radical reactions (Bio-flavored course at UMM covers chapters 10-17 plus NMR) Chapter 12: Acyl substitution reactions Section 1: Introduction to carboxylic acid derivatives and the nucleophilic acyl substitution reaction Section 2: Acyl phosphates as activated carboxylic acids Section 3: Thioesters Section 4: Esters Section 5: Nucleophilic acyl substitution reactions involving peptide bonds Section 6: Activated amide groups Section 7: A look ahead: acyl substitution reactions with a carbon or hydride nucleophile Section 1: Introduction to carboxylic acid derivatives and the nucleophilic acyl substitution reaction A: Carboxylic acid derivatives and acyl groups B: The nucleophilic acyl substitution reaction C: The relative reactivity of carboxylic acid derivatives Section 12.2: Acyl phosphates as activated carboxylic acids A: Glutamine synthetase B: Asparagine synthetase C: Glycinamide ribonucleotide synthetase D: Synthetic parallel - activated carboxylic acids in the lab Section 12.2: Acyl phosphates as activated carboxylic acids A: Glutamine synthetase B: Asparagine synthetase C: Glycinamide ribonucleotide synthetase D: Synthetic parallel - activated carboxylic acids in the lab Section 12.3: Thioesters A: Introduction to thioesters and Coenzyme A B: Activation of fatty acids by coenzyme A - a thioesterification reaction C: Transfer of fatty acyl groups to glycerol: a thioester to ester substitution D: More transthioesterification reactions E: Hydrolysis of thioesters Section 12.3: Thioesters A: Introduction to thioesters and Coenzyme A B: Activation of fatty acids by coenzyme A - a thioesterification reaction C: Transfer of fatty acyl groups to glycerol: a thioester to ester substitution D: More transthioesterification reactions E: Hydrolysis of thioesters Section 12.4: Esters A: Nonenzymatic esterification: synthesis of ‘banana oil’ B: Nonenzymatic ester hydrolysis and the soap-making process C: Enzymatic ester hydrolysis: acetylcholinesterase and sarin nerve gas D: More enzymatic ester hydrolysis: lipase, the resolution of enantiomers, and dehalogenation E: Transesterification: the chemistry of aspirin and biodeisel Section 5: Nucleophilic acyl substitution reactions involving peptide bonds A: Formation of peptide bonds on the ribosome B: Hydrolysis of peptide bonds: HIV protease C: The chemical mechanism of penicillin Section 12.7: A look ahead: acyl substitution reactions with a carbon or hydride nucleophile Other features of OCBE textbook/course • Chapter on phosphates, phosphoryl transfer reactions • Emphasis on importance of aldol/Claisen reactions in biological carbon-carbon bond formation and cleavage • Eliminations: introduced later (chapter 14), focus on E1cb mechanism • Sections on thiamine diphosphate and and pyridoxal phosphate coenzyme mechanisms • Diversity of electrophilic reactions • Redox: focus on nicotinamide- and flavin-dependent hydrogenation/dehydrogenation reactions • Section on disulfide-dithiol redox chemistry, roles of glutathione and lipoamide My hopes for final versions of the new MCAT and MCAT preview • In lists of organic reactions, stress biological relevance, give examples • Rethink references to purely synthetic chemistry (emphasize small set of synthetic ‘tools’, understanding of retrosynthetic strategy) Acknowledgements University of Minnesota, Morris Delmar Larsen, Chemwiki project at UC-Davis