Chem 3.5 #8 Acids

4.
3.
5.
2.
CHEMISTRY 3.5 Name:
WORKSHEET EIGHT ORGANIC CHEMISTRY
Carboxylic acids and their derivatives #1
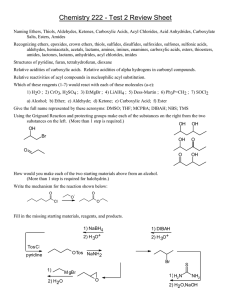
1. Write the names of the following compounds.
(a) CH
3
(CH
2
)
6
COOH
(b) CH
3
CH
2
COOH
Give the condensed structural formulae of the following compounds.
(a) Methanoic acid
(b) Butanoic acid
The carboxylic acids have higher boiling points than alcohols and the lower members are soluble in water.
Explain both these properties in terms of intermolecular attractions.
(a) Write an equation for the reaction of ethanoic acid with water.
(b) Is ethanoic acid a strong acid or a weak acid?
(c) Describe, with an equation, what would take place if magnesium ribbon was placed in ethanoic acid.
(d) Write an equation for the reaction of ethanoic acid with ammonium hydroxide solution.
Carboxylic acids can react with alcohols to make esters.
(a) What is the name given to this type of reaction?
(b) Explain the dual function of the concentrated sulfuric acid reagent.
6.
(c) Write an equation for the reaction between ethanoic acid and propanol under appropriate conditions to produce an ester.
Draw structural formulae for the following acyl chlorides.
7.
(a)
(b)
Ethanoyl chloride.
Butanoyl chloride.
Why do acyl chlorides fume in moist air?
(a) Acyl chlorides are produced from carboxylic acids. Give the formulae of the three reagents that can be used.
8.
(b) Acyl chlorides are very reactive and the chlorine atom is easily replaced. They can be used to make more stable acid derivatives such as:
(c) Write equations for the reaction of ethanoyl chloride with:
(i) Water.
(ii) Ethanol.
(iii) Methylamine.
(iv) Ammonia
9.
(d)
Write the names of the following compounds.
In the reactions (ii) to (iv) in (c), what is the common product?
(a) CH
3
CH
2
COOCH
2
CH
3
(b) CH
3
COOCH
2
CH
2
CH
3
(c) CH
3
CH
2
CH
2
COOCH
3
10. Write the condensed structural formulae for the following compounds.
(a) Methylethanoate
(b) Propylpentanoate
(c) Butylmethanoate