Goal 5 and 6 Review Worksheet
advertisement

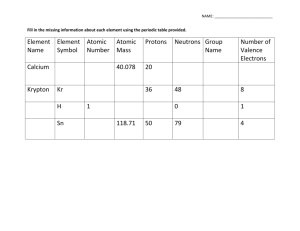
Goal 5 and 6 Review Worksheet Write the name of the Scientist responsible for each model 1. _____________________________ 2. ______________________________ + - + - + + + + - Empty space + 3. Iodine is a halogen found in group 17 & period 6. How many valance e- does iodine have? a. 6 b. 7 c. 11 d. 17 4. Radium is an element found in group 2 and period 7. How many energy levels does an atom of radium contain? a. 1 b.6 c. 7 d. 8 5. Francium is an element found in group 1 and period 7. How many valence electrons does an atom of francium contain? a. 1 b. 6 c. 7 d. 8 6. Look at the element in the picture. What are the element’s name, atomic number, and number of electrons? 19 a. K, 19, 19 K Potassium b. K, 39.098, 19 39.098 c. Potassium, 19, 19 d. Potassium, 39.098, 39 7. Which element is stable, exists as a single atom in air, and has a stable outer energy level? a. Hydrogen b. Argon c. gold d. oxygen 8. What type of element typically loses electrons to form a bond? a. Non-metal b. noble gas c. metalloid d. metal 9. At what ratio will potassium and sulfur form a binary ionic compound? a. 1:1 b. 1:2 c. 2:1 d. 3:2 10. Which one of the following formulas is correct? a. KC2H3O2 b. Mg(PO4)2 c. K(OH)2 d. K2MnO4 11. What is true in every balanced chemical equation? a. All coefficients have to be multiplies of 2 b. Subscripts are changed c. Atoms are created when products are formed d. Mass has been conserved 12. What is the coefficient on Al2O3 when the equation describing the following synthesis reaction is balanced? Al + O2 Al2O3 a. 1 b. 2 c. 3 d. 6 13. An isotope of Carbon is C-14. Its atomic number is 6. How many neutrons are found in C-14? a. 6 b. 8 c. 14 d. 20 14. Cesium is a Group I alkali metal. It is found in Period 6 on the Periodic Table. What kind of ion will a cesium atom form to reach chemical stability? a. +1 b. -1 c. +6 d. -2 15. Where is the majority of the mass located in the atom? a. nucleus b. protons c. neutrons electron cloud 1) Write a correct formula for Potassium iodide 2) Name NH4OH Write equations, balance them and tell the type of reaction for the following reactions: 3) The decomposition of hydrogen peroxide to form water and oxygen 4) Sodium Hydroxide combines with Hydrochloric Acid to form a neutralization reaction 5) *just tell what type of reaction this is* Running an electrical current through water to produce carbon dioxide and water 6) Reacting iron with oxygen to produce Iron (III) oxide 7) Burning magnesium in the presence of oxygen to produce magnesium oxide Balance the following and tell what type of reaction is taking place *Diatomic Molecules 1. ___________ Na (s) + Cl (g) 2. _________ Na (s) + H2O(l) NaOH(s) 3. _________ Mg (s) + O (g) MgO (aq) 4. _________ AgNO3 (s) + Cu (s) Cu(NO3)2 (s) 5. _________ HCl (aq) + 6. _________ FeS (aq) + HCl (aq) P O 7. _________ + NaCl(s) KOH(aq) P2O5 + H (g) + Ag (s) KCl (s) FeCl2 (s)+ + H2S (l) H2O(l)