hw2012_01
advertisement

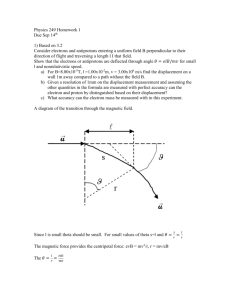
Physics 249 Homework 1 Due Sep 14th 1) Based on 3.2 Consider electrons and antiprotons entering a uniform field B perpendicular to their direction of flight and traversing a length l I that field. Show that the electrons or antiprotons are deflected through angle 𝜃 = 𝑒𝑙𝐵/𝑚𝑣 for small l and nonrelativistic speed. a) For B=8.00x10-5T, l =1.00x10-2m, v = 3.00x106 m/s find the displacement on a wall 1m away compared to a path without the field B 1m. b) Given a resolution of 1mm on the displacement measurement and assuming the other quantities in the formula are measured with perfect accuracy can the electron and proton by distinguished based on their displacement? c) What accuracy can the electron mass be measured with in this experiment. 2) 3.18 Calculate the average energy per mode of oscillation for a long wave length 𝜆 = 10ℎ𝑐/𝑘𝑇 and a short wavelength 𝜆 = 0.1ℎ𝑐/𝑘𝑇 based on the quantum formula and compare your result with the classical expectation kT. Express your answers as numbers times kT. 3) 3.32 The longest wavelength of light that will cause emission of electrons from cesium is 653nm. a) Compute the work function for cesium. b) If the light of 300nm were to shine on cesium, what would be the energy of the ejected electrons. Give answers in units of eV 4) Based on 3.46 In his first e/m experiment Thomson measured q/m of the electrons by collected them in an insulated beam stopper and measuring the total collected charge Q and the temperature rise deltaT in the beam stopper (known as calimetric energy measurement). Derive a formula for q/m in terms the specific heat, c, and the mass, M, of the beam stopper, the velocity of the electrons and the measured values. 5) 3.56 Estimate the time lag (expected classically but not observed) for the photoelectric effect. Assume a point source emits 1W of light energy uniformly in all directions. For an atom with a work function of 2eV at a distance of 1m how long does it take for the required energy to be absorbed. Assume a reasonable size for the atom and that all energy hitting the atom is absorbed.