Metal Reactivity Lab: Group 1 & 2 Reactions
advertisement

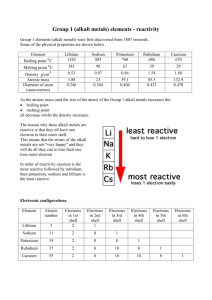
Reactivity of Metals in the Periodic Table In this experiment, you will be looking at the reactions of three metals in Group 1 (lithium, sodium, and potassium) and two metals in Group 2 (magnesium ad calcium). Only displacement reactions will be used in this investigation. A + BC AC + B Element A can displace element B from the compound BC only if element A is more reactive than element B. If A is less reactive than B, then no reaction occurs. Safety Precautions: Safety glasses must be worn at all times. Do not touch metals with your hands because they are extremely reactive with moisture. MATERIALS: safety Glasses tongs tweezers mineral oil sharp edge (knife or scoop) paper towel Petri dish large beaker distilled water wire gauze square samples of lithium sodium potassium magnesium calcium PROCEDURE: 1. Draw a suitable chart to record all your observations. Your chart should include qualitative observations before, during and after the reaction. 2. Transfer a small piece of lithium into a Petri dish containing mineral oil. 3. Using a knife or similar sharp edge, remove the surface layer from one side of the piece of lithium. Observe the fresh surface. 4. Fill the beaker about half full of water. 5. Cut a small piece (about 2 mm cubed) of the element. Remove any oil with paper towel and drop the piece of the element into the beaker. Immediately cover the beaker with the wire gauze. Record observations of any chemical reaction. 6. Place a piece of litmus paper into the solution in the beaker. 7. Repeat steps 2 through 5 using sodium, potassium, magnesium and calcium. QUESTIONS: 1. a) Potassium, sodium, and lithium are members of Group 1 of the periodic table. What evidence suggests that the Group 1 metals react with water in the same way? (Hint: Write the electron configurations of the three metals.) b) Did all three metals of Group 1 react with water at the same speed? Explain. 2. a) Based on your observations, arrange lithium, sodium and potassium in order from most reactive to least reactive. b) If this list could be extended to all the metals in Group 1, what do you think the order would be, from most reactive to least reactive? 3. Based on your observations, is the solution tested using the litmus paper acidic or basic? Explain. 4. Based on your observations for magnesium and calcium, place all the metals in Group 2 in order from most reactive to least reactive. 5. Complete the following word equations for the reactions of the metals with water. Place a slash through the arrow if these is no reaction. (Hint: The chemical name for water is hydrogen hydroxide.) a) lithium + water b) sodium + water c) potassium + water d) magnesium + water e) calcium + water 6. Translate the word equations from #5 into balanced chemical equations – states should also be included!!! 7. Compare potassium to calcium, and sodium to magnesium. Which group of metals is more reactive, Group 1 or Group 2? 8. Which do you think is the most reactive metal in the periodic table? Explain your answer.