Group 1 - The Alkali Metals
advertisement

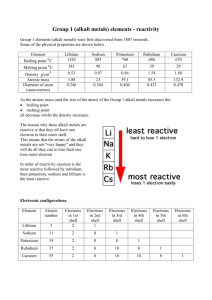
Group 1 - The Alkali Metals lithium Li sodium Na potassium K rubidium Rb cesium Cs francium Fr The Group 1 contains 7 elements, but one of them cannot be registered to this group, because it isn´t a metal. It ´s Hydrogen H2. We can talk long time about this group, but we try to choose some interesting facts about it. All the Group 1 elements are silvery-coloured metals. They are soft, and can be easily cut with a knife to expose a shiny surface which dulls on oxidation. Next interesting attribute is, that five of them give a flame a specific colour. Let´s look at it… Li Lithium was discovered in 1817 by Johann Arfvedson (Sweden). Lithium is the lightest of all metals, with a density only about half that of water. It does not occur free in nature; combined it is found in small units in nearly all igneous rocks and in the waters of many mineral springs. It reacts with water, but not as vigorously as sodium. Lithium imparts a beautiful crimson color to a flame, but when the metal burns strongly, the flame is a dazzling white. Among others lithium compounds are used in dry cells and storage batteries. Na Sodium was long recognized in compounds only, sodium was first isolated by Sir Humphrey Davy in 1807 by electrolysis of caustic soda. Sodium is the fourth most abundant element on earth. Imparts a beautiful crimson color to a flame (Metallic sodium is vital in the manufacture of esters and in the preparation of organic compounds.) Sodium metal should be handled with great care. It cannot be maintained in an inert atmosphere and contact with water and other substances with which sodium reacts should be avoided. K Potassium was discovered in 1807 by Sir Humphrey Davy, too , who got it from caustic potash (KOH); this was the first metal isolated by electrolysis. The metal is the seventh most abundant of the earth's crust. Potassium is also found in the ocean, but is present only in relatively small amounts, compared to sodium. Potassium is an essential constituent for plant growth and is found in most soils. Rb, Cs, Fr Rubidium and Cesium were recognized by Bunsen and Kirchoff in second half of 19th century, Francium was discovered in 1939 in Paris (Marguerite Perey). There are some interesting facts about these elements: Rubidium, alcalic metal, can be liquid at room temperature. Cesium is the most electropositive and most alkaline element, for example it reacts explosively with cold water, and reacts with ice at temperatures above -116C. Francium is the heaviest known member of the alkali metals series. QUIZ: 1.How many elements is in the Group 1? a) 7 M b) 8 K c) 6 A d) 6 and half D 2. Lithium compounds are used.. a) in the manufacture of Teflon b) as a reducing agent in preparing other metals. c) in dry cells and storage batteries. d) in manufacture of Coca Cola U I E X 3. What rank is Sodium in abundance on earth? a) fiveth b) fourth c) sixth d) fourty-seventh 4. Where can we find a few of Potassium? a) in air b) in ocean c) in fruit d) in a bathrom 5. Which element was recognized the latest? a) Fr b) Cs c) Rb d) each were recognized in the same time 6.How did you like this presentation? a) It was good. b) It was realy good. c) Is it the last question? d) It was famous!! Words: contain R T D S E A U V L K A C S S S S skládat se (z čeho), obsahovat expose shiny surface colour flame attribute igneous rock spring fused appearance vigorously crimson impart dazzling among others dry cell storage battery recognize abundance maintain comparison předložit,odhalit lesklý povrch barvit plamen vlastnost, znak vyvřelá hornina pramen roztavený výskyt, zjevení silně karmínový propůjčit, předat oslnivý mimo jiné baterie akumulátor poznávat zastoupení udržovat porovnání