Alkali Metals SOP
advertisement

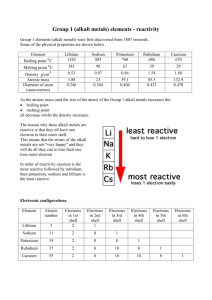
Standard Operating Procedure ________________________________________________________ Read the EH&S Standard Operating Procedures Fact Sheet before filling out this form. Print out the completed form and keep a readily accessible hard copy in the lab (also keeping an electronic copy is highly recommended). ______________________________________________________ Date: November 21, 2010 SOP Title: Alkali metals – Li, Na, K Principal Investigator: Richmond Sarpong Room and Building: 841A Latimer Hall Lab Phone Number: (510) 643-2485 Section 1 – Process The handling and usage of alkali metals, specifically lithium, sodium, and potassium. Section 2 – Hazardous Chemicals Lithium, sodium, and potassium metal. Section 3 – Potential Hazards Alkali metals react very vigorously with water resulting in the formation of hydrogen gas. This gas can then spontaneously ignite, causing fires. Additionally, if inhaled, the dust of alkali-metal oxides can cause damage to the mucous membranes and upper respiratory tracts. Contact of any of these metals with the skin or eyes may results in burns. Caustic oxides are formed as the metals burn. Of these three, potassium and the liquid alloy of potassium and sodium are the most reactive, while lithium is the least reactive. Section 4 – Approvals Required Use of lithium, sodium, or potassium metal requires proper training and demonstration of correct technique by an appropriate lab member. The MSDS sheets should also be consulted before first use. These metals must not be used when working alone. Section 5 – Designated Area Lithium, sodium, and potassium metals should only be used in a dry environment away from sparks or any source of ignition. Good ventilation and access to a dry chemical or dry powder fire extinguisher are also necessary. The area where the metals will be handled should be free of other chemicals and flammable objects. Section 6 – Special Handling Procedures and Storage Requirements Stored in a dry, cool place away from any source of ignition. Store under paraffin oil, mineral oil, or kerosene . When cutting or weighing out sodium or potassium, they must be kept under hexanes or toluene as much as possible to prevented them from reaction with the moisture in the air. The container holding the metal must be kept closed, and the amount of material exposed to the air kept to a minimum. N2 is not an inert gas for lithium, as lithium nitride is formed and can also react violently with water. Lithium should therefore not be kept under nitrogen for a prolonged period of time (use Ar instead). Section 7 – Personal Protective Equipment Respiratory protection Where risk assessment shows air-purifying respirators are appropriate use a full-face particle respirator type N100 (US) or type P3 (EN 143) respirator cartridges as a backup to engineering controls. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Hand protection Handle with gloves. Eye protection Safety glasses. Skin and body protection A flame-retardant lab coat must be worn while handling these compounds. Hygiene measures Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday. Section 8 – Engineering/Ventilation Controls Ensure adequate ventilation. Section 9 – Spill and Accident Procedures EHS&S and Campus Safety should be called in the event of a large spill or fire: (Emergencies: 911 or 642-3333 from a cell phone; Non-emergencies: EHS&S 642-3073, UCPD 642-6760) DO NOT use water to attempt to extinguish a reactive material fire as it can actually enhance the combustion. Do not use combustible materials (paper towels) to clean up a spill, as these may increase the risk of igniting the reactive compound. Soda ash (powdered lime) or dry sand should be used to completely smother and cover any small spill that occurs. A container of soda ash (powdered lime) or dry sand should be kept within arm’s length when working with a reactive material. If anyone is exposed, or on fire, smothering the fire is a better course of action than washing with water because water can fuel the fire. Class D extinguishers are recommended for combustible solid metal fires. Call 9-1-1 for emergency assistance and for assistance with all fires, even if extinguished. Section 10 – Waste Disposal After completion of the cutting process, the weigh boat or other weighing container should be rinsed carefully with a solvent which will react with the excess metal much more slowly than with water (i.e. methanol, isopropanol). Disposal of Pyrophoric Reagents Any container with a residue of reactive materials should never be left open to the atmosphere. Any unused or unwanted reactive materials must be destroyed by transferring the materials to an appropriate reaction flask for hydrolysis and/or neutralization with adequate cooling. Section 11 - Decontamination All materials – disposable gloves, wipers, bench paper, etc. - that are contaminated with pyrophoric chemicals should be disposed as hazardous waste (after appropriate quenching of the compound, see section 10). Training Documentation Name (Printed) Signature Date