Data table for Chemical Properties of Some Metals
advertisement

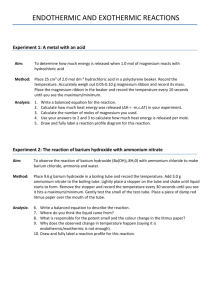
Chemical Properties of Some Metals Introduction: All metals have certain properties in common. In this experiment, you will test several metals and their reactivity with water and hydrochloric acid. To organize the information in this experiment, a chart of your observations is required. The metals to be tested are sodium, potassium, calcium, magnesium, aluminum and lithium. Purpose: In this experiment, I will determine the reactivity of six different metals with water and acid. Materials: Beakers, Test Tubes, Hydrochloric Acid (HCl), splints, water, sodium, potassium, calcium, lithium, aluminum, magnesium Procedure: Part 1 - REACTIVITY WITH WATER CAUTION: Sodium and potassium react vigorously with water; follow the directions carefully. Wear goggles at all times! 1. 2. 3. 4. 5. Obtain a piece of freshly cut sodium abut ½ the size of a match head from your teacher. Do not touch it with your hands. Place it on a piece of dry paper toweling. Using forceps place the sodium into a 250 ml beaker that is ½ full with tap water and immediately cover it with wire gauze. What appears to happen to the sodium? Would you call the rate of reaction slow, moderate or rapid? If a gas is released it is hydrogen gas. Record your observations in the data table. Pour the contents of the beaker in the drain and rinse. Follow the same procedure as above with a piece of potassium. Again, if a gas is produced, it is hydrogen gas. Record your observations. Empty the beaker contents and rinse the beaker. Set up four medium sized test tubes in a test tube rack and fill each with about 2 ml of tap water. In the first test tube place a piece of calcium metal. You may touch the calcium if your hands are DRY. The gas produced is hydrogen gas. As the gas is being released at a rapid rate, attempt to “explode” the hydrogen gas by holding a burning splint to the mouth of the test tube. Record your observations. Pour out the contents of the test tube in the sink and rinse out the test tube. One at a time, add a small piece of each of the other metals (lithium, magnesium, and aluminum) to separate test tubes with 2 ml of tap water. Test the gas with the burning splint, if the reaction produces a sufficient quantity. Record your observations. Pour out the contents of the tubes and rinse. Part 2 – REACTIVITY WITH DILUTE ACID NOTE: The reaction of sodium and potassium with dilute acids is too dangerous to try in the high school laboratory. These will be shown to you on video. Please record your observations from the video. 1. Using the four rinsed-out test tubes from part 1, fill each tube with 2 ml of 6M HCl. To each test tube add, one at a time, a small amount of calcium, magnesium, lithium and aluminum. If a gas is produced it is hydrogen gas. You can test the gas with a burning splint if you wish. Record your observations. Empty the contents of the test tubes in the sink and rinse out the test tubes with water. Data table for Chemical Properties of Some Metals Metal Reaction with water Reaction with Acid Sodium Potassium Calcium Lithium Magnesium Aluminum Questions: 1. a) Draw a diagram showing the positions of each element in relationship to each other (hint: look at the periodic table). b) Draw an arrow from the top left side to the top right side of the diagram. c) Draw an arrow from the top left side to the bottom left side of the diagram. 2. Discuss what trends you see as you move across a period with how these metals reacted with acid? With water? 3. Discuss what trends you see as you move down a group with how these metals reacted with acid? With water? 4. Which group reacts most readily with acid? With water? Give an explanation using electron configuration as to why this is the case. 5. Francium is the most reactive metal of all known metals. Explain this using the electron arrangement of this metal.