SNC 1D- Chemistry Unit Test Review Use the following to help you
advertisement

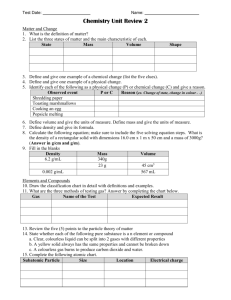
SNC 1D- Chemistry Unit Test Review Use the following to help you review for the upcoming Chemistry Unit Test. Test Date: ___________________ Key Terms – know the definitions for the following terms Pure substance Element Compound Homogeneous mixture Lustre Brittleness Malleability Combustibility Heterogeneous mixture Volume Form Ductility Proton Density State Texture Viscosity Neutron Mass Clarity Hardness Conductivity Electron Main concepts covered- Use these topics to help you make study notes about the material covered in class. 1. The Nature of Matter- Changes of state diagram. List the 5 points of the particle theory of matter. Be able to classify matter as a pure substance (element/compound) or mixture (solution/mechanical mixture) and explain your classification 2. Physical and Chemical Properties of Matter- Identify a property as physical or chemical, qualitative or quantitative. Know the physical and chemical properties from the notes and be able to use these descriptors to describe matter. Be able to provide examples of each type of property. 3. Mass, Volume and Density- Understand how these quantitative properties are related. Know the definitions of mass, volume and density. Be able to use the density equation to solve for density, mass or volume. Understand how to show your work using the GRASS method. 4. Physical and Chemical Change- What is the difference between a physical and chemical change? What are the three types of physical changes? What are the five indicators of chemical change? Be able to classify an observation as a physical or chemical change. 5. Atomic Theories- What is the discovery/idea of: Democritus, Aristotle, Dalton, Thomson, Rutherford, Chadwick and Bohr? How does each scientist’s idea build on the previous model(s)? Describe Rutherford’s gold foil experiment. 6. Atomic Structure- Where are the following subatomic particles located: protons, neutrons, electrons? What is the charge and mass of the electron, proton and neutron? Draw a diagram of the atom showing the nucleus, protons, neutrons, electrons and electron levels. 7. Bohr-Rutherford Diagrams- Be able to draw the Bohr-Rutherford Diagram for each of the first 20 elements in the periodic table (given a periodic table). 8. Trends in the Periodic Table- Be able to label the main groups (halogens, noble gases, alkali metals and alkaline earth) on the periodic table. Which groups are highly reactive? Which group is stable? How does the number of valence shell electrons affect reactivity? Be able to identify each group as a metal or non-metal. 9. Gas Tests- What is the test for hydrogen gas? Explain what will happen if hydrogen gas is present. What is the test for oxygen gas? Explain what will happen is oxygen gas is present. What is the test for carbon dioxide gas? Explain what will happen if carbon dioxide gas is present. 10. Counting Atoms and Building Molecules- Know how to identify what elements are present in a compound and how many of each type of atom is present. Be able to identify an element and a compound given the chemical formula. Review Questions 1. Practice Matching Questions-pg 289 # 41 and 42 2. Practice Multiple Choice Questions- pg 288 # 1-12 3. Practice Short Answer Questions - pg 202 # 1, 2, 3, 7, 10, 11, 12, 16-19 - pg 248 # 5, 6, 7, 10, 11, 12, 15 - pg 290 # 43, 44, 51, 56, 75