work sheet 3
advertisement

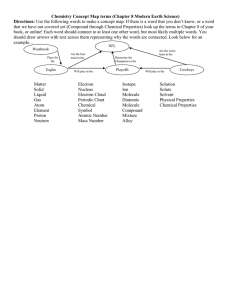
`المملكة العربية السعودية Kingdom of Saudi Arabia وزارة التعليم العالي Ministry of Higher جامعة المجمعة Education عمادة السنة التحضيرية Majma'a University Preparatory Year Deanship WORK SHEET (3) Question (1):Choose the correct answer of the following:1) H2 is : a- Compound b- Heterogeneous mixture c - Element d- Homogeneous mixture 2) Sand with iron fillings is : c- Compound d- Heterogeneous mixture c - Substance d- Homogeneous mixture 3) Water is : a- Molecular b- Pure substance c- Homogeneous mixture d- both a and c 4) Pure substance is : a- Compound b- Molecular c- element d- both a and c 5) All of the following is physical change except: a- Ice melting or water freezing c- evaporation of water d- metal tarnishes in air b- Melting of metals 1 6) All of the following is chemical properties except : a- Iron rusting c- metal tarnishes in air b- Magnesium reacts with d- Magnesium metal is grey hydrochloric acid 7) One of the following is extensive property : a- Melting point b- Volume c – Boiling point d – Density 8) One of the following is intensive property : a- Length c– mass b- Volume d – Density 9) The SI unit of length is the: a- Meter b- Foot c- yard d- liter 10) All of the following are Diatomic molecules except : a) He b) HCl c) F2 d) CO 11) All of the following are Monoatomic molecules except : b) He b) HCl c) Ar 12) The derived SI unit of the following is: a- K b- m2 c- mm 2 d) C d- s 13) The derived SI unit of the following is: b- m b- mol c- ms 14) a- 15) a- micro = …………. : 109 b- 10-6 Nano = …………. 109 c- 10-3 d- L d- 10-9 : b- 10-6 c- 10-3 d- 10-9 Gega = ………….. : 109 b- 106 c- 10 -9 d- 10-6 17) a- 346 73 K = ………… oC : b- 226 c- 200 d- 32 18) 47oC = ………… K : 16) a- a- 320 19) a- 38 b- 226 100 °F= ………….°C: b- 39 20) 77K= ……………°C: a- -196 b- -169 c- 273 d- 32 c- 40 d- 45 c- 231 d- 230 3 21) Glass has a density of 2.2 g/cm . the volume occupied by 22g of glass =………….. a- 10 cm3 b- 48.44 cm3 c- 0.1 cm3 d- 100 cm3 3 22)The formula of a compound formed between Mn4+ and S2- is: a- MnS2 b- Mn2S4 c- Mn2S d- Mn4S2 23) The formula of a compound formed between Na+ and N3- is: a- Na3N b- NaN3 c- NaN d- Na3N3 24) The formula of a compound formed between Fe3+ and O2a- Fe2O3 b- Fe3O2 c- FeO d- no one 25) The formula of compound formed between Cu+1 and O2a- CUO b- CU2O c- CUO2 d- no one 26) Mass number is : a- No of proton b- No of proton and electron neutron c- No of electron d- No of proton and 27) Atomic number is : a- number of proton b- number of electron neutron c- both a and b d- number of 28) All of the following are characteristics of metals except: a- Malleable c- conducted to heat b- Ductile d- gain electron 4 29) Cation is : a- Positively charge ion b- Negatively charge ion c- a and d d- atom lose electron 30) Anion is : a- Negatively charge ion b- Form from nonmetal c- all answer d- atom gain electron 31) The number of neutrons in a- 19 d- 18 32) 𝟑𝟗 𝟏𝟗𝑿 are : b- 39 c- 20 The number of neutrons in a- 11 b- 12 d- 23 33) The number of electron in 𝟐𝟑𝟓 𝟗𝟐𝑿 a- 92 b- 235 𝟐𝟑 𝟏𝟏𝑿 are : c- 10 are: c- 183 d- 143 34) a) 7 How many significant figures does the number ( 13. 090) has: b) 6 c) 5 d) 4 35) a) 3 How many significant figures does the number ( 130,000) has: b) 4 c) 5 d) 6 34) How many significant figures does the number ( 0.001309) has: a) 7 b) 6 c) 5 5 d) 4 Question (2):Give an one example from the following set ( , NO3¯, Br , Cl¯ , Na+ ) for the following: 1 ) Anion ……………………… , 𝟏𝟎𝟗 𝟒𝟕𝑨𝒈 , SO42¯ , K , 𝟏𝟎𝟕 𝟒𝟕𝑨𝒈 3 ) Metal ………………………………. 2 ) Isotopes ………………… and …………………. 4 ) Polyatomic ion ……………..………… 5) cation …………………6) nonmetal………………. 6 Question (3):Write the name each of the following compounds : 1 - NF3 ……………………………………………………………………………………… 2 - KCl ……………………………………………………………………………………… 3- FeO ……………………………………………………………………………………… 4- Na2CO3∙10H2O : …………………………………………………………………………. 5- CaSO4 . 2 H2O ……………………………………………………………………………………… 6- (NH4)3 PO4 ……………………………………………………………………………………… 7- PCL5 ……………………………………………………………………………………… 8- CUO ……………………………………………………………………………………… 9- NaBr ……………………………………………………………………………………… 10- CS ……………………………………………………………………………………… 11- CaO : ……………………………………………………………………………………... 7