EXPERIMENT
advertisement

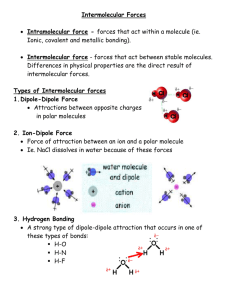
EXPERIMENT - 6 Solubility and Conductivity INTRODUCTION- One of the important physical properties of a chemical compound is its solubility. When a compound is soluble in another, the two form a solution. A solution is a homogeneous mixture that generally contains a large amount of one component (solvent) into which a small amount of another component (solute) is dissolved. You probably have observed that some pairs of liquids, such as oil and water do not mix (immiscible liquids), whereas others, such as alcohol and water do mix readily (miscible liquids). Two compounds that do dissolve in one another are said to be soluble and form a solution as described above. Those that do not dissolve are referred to as insoluble. The common description used to describe this phenomenon is “like dissolves like”. “Like dissolves like” is a general rule frequently stated by chemist. This rule means that the more similar substances are in their properties, then the more likely the substances are to dissolve in one another. Polar compounds are more soluble in polar solvents and less soluble in nonpolar solvents; and nonpolar compounds are more soluble in nonpolar solvents and less soluble in polar solvents. A molecule like water, which has an angular shape (bent) the bonds form an angle of 105o and which has polar H-O bonds, has a partial positive charge at the hydrogen end and a partial negative charge at the oxygen end. Water has two poles (dipole); it is a polar molecule. The polar nature of the water molecule is largely responsible for its ability to dissolve ionic compounds and other polar covalent compounds. Many chemical reactions and most biochemical reactions take place in water. On the other hand, molecules such as cyclohexane (C6H12), carbon tetrachloride (CCl4), and dichloromethane (CH2Cl2), which have polar C-H, C-Cl, and C-C bonds but a symmetrical distribution of the bond are not dipole molecules. They are nonpolar molecules. Kerosene, oil, and gasoline are also nonpolar compounds. Soluble ionic compounds contain positive and negative ions that are attracted to polar compounds like water but not to nonpolar compounds. If ionic compounds dissolve in water, the charged ions are free to move about in the solution independent of each other. These solutions will conduct an electric current. An electric current is the movement of charged particles, which are electrons in a solid such as metal or charged ion in a solution. Aqueous solutions of some substances will conduct electricity. These solutions are called electrolytes. Other substances whose solutions will not conduct an electric current are called nonelectrolytes. 1 When an electrolyte is dissolved in water, ions are produced. Ions are responsible for conductivity. We will distinguish between two types of substances that produce ions in solution. Strong electrolytes form aqueous solutions that conduct electricity very easily. The conductivity is high because strong electrolytes in solution exist almost entirely as ions. Weak electrolytes, on the other hand, form solutions that are poorer conductors. In solution, weak electrolytes exist primarily as molecular substances with only a few ions. Electrolytes may be ionic solids such as sodium chloride, NaCl, or they may be covalent compounds that react with the solvent to produce ions in solution. Ionic: NaCl (aq) Na+ (aq) + Cl+ (aq) Covalent: HCl (g) + H2O (l) H3O + or H +(aq) + Cl- (aq) If both ions in an ionic compound are charged (more positive or more negative) and small in radius (ionic size), these compounds tend to be less soluble. Review solubility rules for solubility of compounds in water. Electrolytes are vital constituents of body fluids. Hydrochloric acid secreted in the stomach aids in digestion. Solutions, which bathe cell walls, need to be in proper electrolytic balance to control the passage of fluids through the walls. Nerve impulses depend on ions to provide electrical conductivity. We can easily test all solutions for their conductivity by placing them in an electrical circuit in such a way so that a light bulb glows brightly for strong electrolytes, dimly for weak electrolytes, and does not glow at all for nonelectrolytes. Strong electrolytes (SE) sodium chloride, NaCl potassium acetate, KC2H3O2 nitric acid, HNO3 sodium hydroxide, NaOH sulfuric acid, H2SO4 Weak electrolytes (WE) acetic acid, HC2H3O2 carbonic acid, H2CO3 ammonium hydroxide, NH4OH (ammonia) sulfurous acid, H2SO3 Nonelectrolytes (NE) ethanol, C2H5OH methanol, CH3OH acetone, CH3OCH3 glycerin, C3H5 (OH)3 ethylene glycol ,C2H4(OH)2 (antifreeze) In this experiment you will test the solubility of several compounds in water and cyclohexane. From the solubility properties, you will predict whether the compound is like water (polar) or like cyclohexane (nonpolar). The solubilities of many substances, not gases, increase with increasing temperature. An increase in pressure increases the solubility of gases but affects solid and liquid solubilities very little. In addition to being soluble in water, you will test such solutions for their conductivities using a conductivity tester, light emitting diode (LED), to indicate the electrical current from produced ions. 2 Experimental Procedure Part I – Solubility Select ten test tubes (smallest test tubes) and add a small amount of substance in each, (for solids use a sample less than the size of a match head and for liquids use a few drops). Add about 1 ml (15-20 drops) of distilled water (or tap water) to each of the ten test tubes. Shake each test tube well and test each substance one at a time for water solubility. Record your observations on the report form as soluble (S), insoluble (INS), or slightly soluble(SS). Part II – Conductivity CATION- The bare electrodes are a hazard! DO NOT TOUCH the electrodes when the apparatus is plugged in. Your skin will conduct an electric current and cause a SHOCK. Select one solution at a time to test. Place the electrodes (diode) in the solution and observe the light bulb. Identify the solution as strong electrolyte (SE), weak electrolyte (WE), or a nonelectrolyte (NE). 3 EXPERIMENT – 6 REPORT FORM Name ___________________________ Instructor ________________________ Date ______________ Part I – Solubility Substances Solubility in water 1. sucrose 2. ammonium chloride 3. naphthalene 4. sand (silicon dioxide) 5. sodium chloride 6. vegetable oil 7. mineral oil 8. toluene 9. potassium permanganate 10. ethyl alcohol 4 Solubility in cyclohexane Part II – Conductivity aqueous solutions formula physical states 1. tap water 2. sodium chloride 3. sodium hydroxide 4. potassium nitrate 5. acetic acid (vinegar) 6. rubbing alcohol (isopropanol) 7. hydrochloric acid 8. ammonium hydroxide 9. sugar (sucrose) solution 10. acetic acid + sodium chloride 5 color ionic/covalent EXPERIMENT – 6 Name Pre- laboratory Questions and Exercises Due before lab begins. Answer in space provided. 1. Define the following terms: a. conductivity b. solubility 2. Define strong electrolyte, weak electrolyte, and nonelctrolyte and give one example of each. 3. Give two special safety precautions that must be observed during this experiment. 4. What are the consequences of students in a chemistry lab disposing of organic solvents such as toluene, cyclohexane, and oil in the sink? 5. Arrange the following list of solutions from poorest to best conductors of electricity: hydrochloric acid ammonium hydroxide 6 water toluene EXPERIMENT – 6 Name Post- laboratory Questions and Exercises Due after completing lab. Answer in space provided. 1. Define the following terms: a. polar substance b. nonpolar substance 2. Define miscible and immiscible liquids and give one example of each. 3. Write an equation for the solution of the following in water: a. H2SO4 b. Ca(OH)2 c. sodium acetate, NaC2H3O2 4. What are the factors (at least four) which increase solubility of a solute in a solvent? 5. Predict whether the following compounds are ionic, polar covalent, or nonpolar. a. Na2SO4 _______ e. Acetone, CH3 CO CH3 ________ b. Octane, C8H18 _______ f. Ether ________ c. NaC2H3O2 _______ g. Gasoline ________ d. Methanol, CH3OH _______ h. Carbon tetrachloride ________ 7