Intermolecular Forces and Solubility
advertisement
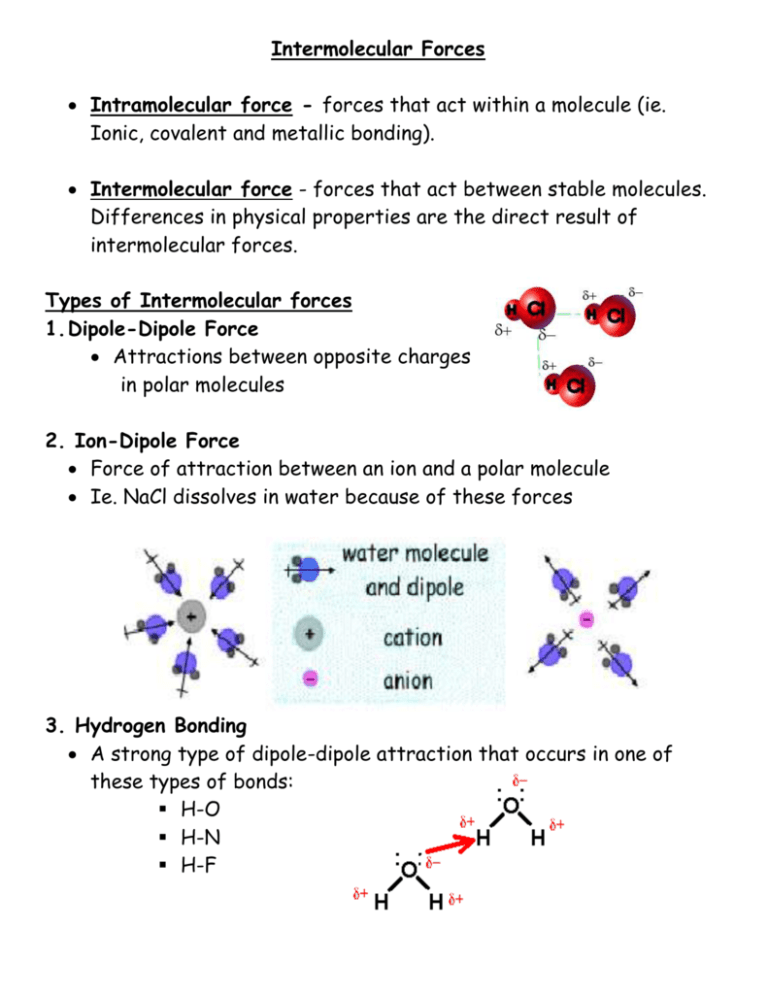
Intermolecular Forces Intramolecular force - forces that act within a molecule (ie. Ionic, covalent and metallic bonding). Intermolecular force - forces that act between stable molecules. Differences in physical properties are the direct result of intermolecular forces. Types of Intermolecular forces 1. Dipole-Dipole Force Attractions between opposite charges in polar molecules 2. Ion-Dipole Force Force of attraction between an ion and a polar molecule Ie. NaCl dissolves in water because of these forces 3. Hydrogen Bonding A strong type of dipole-dipole attraction that occurs in one of these types of bonds: H-O H-N H-F Intermolecular Forces and Solubility “Like dissolves Like” For example polar substances will dissolve in other polar substances and nonpolar in other nonpolar substances. But Why? Solubility of Polar Covalent Substances in Water The partial charges in a polar substance are attracted to the opposite partial charge in water’s polar molecules. As a result water molecules surround the polar molecules, causing them to dissolve. Example methanol (CH3OH) Solubility therefore is the result of a special dipole-dipole attraction called hydrogen bonding. Solubility of Ionic Compounds in Water When a soluble ionic compound dissolves in water the attraction between the two ions is broken (ionized) and the two ions are surrounded by molecules of water. Solubility therefore is the result of ion-dipole attraction. Some ionic compounds are not soluble in water. This is the result of the very strong attractions of their ions. Ie. Silver chloride When the electronegativity difference of a binary compound is small (ie. not ionic or polar), it is likely to be soluble. Factors Affecting Solubility 1. Molecule size Small molecules are more soluble than large molecules because it is easier for solvents to surround these particles. Many larger molecules are also less polar overall therefore intermolecular forces such as hydrogen bonding have less effect. Example: Methanol (CH3OH) vs. Ethanol (CH3CH2OH) 2. Temperature When solids dissolve in a liquid energy is required to break the bonds. Increased temperatures supply this energy. Therefore solubility increases in solids as temperature increases. Intermolecular forces between liquids are not as strong; therefore solubility of liquids is not greatly affected by temperature. Gases already move very quickly. Therefore if the temperature of a solution of a gas dissolved in liquid increases, the gas will gain energy and be less soluble. 3. Pressure and solubility Pressure mainly affects the solubility of gases. Direct relationship – as pressure increases, solubility increases. Pressure acts to keep gases from escaping the solution. Factors that affect solubility of ionic compounds: Some ionic substances such as sodium chloride [NaCl] are soluble in water, while other ionic substances, such as calcium phosphate [Ca3(PO4)2] are insoluble. Ion charge – compounds of ions with large charges tend to be insoluble because increasing the charge, increases the force that holds the ions together. Example: Phosphates PO43- tend to be insoluble while salts of alkali metals (ie. Na+1, K+ etc.) tend to be soluble. Ion size – compounds containing small ions tend to be less soluble than compounds with larger ions of the same charge. Small ions bond more closely together and therefore the force of attraction is much more difficult to break (ionize). Example: Fluoride ions are less soluble than chlorine ions.











