Aqueous Solutions Notes
advertisement

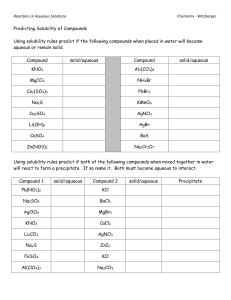
Chapter 6: Chemical Reactions Date:____________ Section 4: Aqueous Solutions - Notes Objectives: Determine if a compound is soluble. Aqueous Solutions: A compound is _____________________ in a particular liquid if it _________________________ in that liquid. A compound is __________________________ if it does ________________________________ in the liquid. An aqueous solution is a ______________________________________ mixture of a substance with __________________. o When ionic compounds dissolve in water, they usually ___________________________ into their component _________. A sodium chloride solution, NaCl(aq), does not contain any ________________units. o Only dissolved ________________ and _________________ are present. Substances (such as NaCl) that completely dissociate into ions in solution are called __________________________________________. Pure water does ______________________________ electricity. Ions in a sodium chloride solution ______________________________ electricity, causing the bulb to ______________. AgNO3(aq) is a ____________________________________ solution. When compounds containing polyatomic ions such as ____________ dissolve, the polyatomic ions dissolve as _________________________. Not all ______________________________________ dissolve in water. o AgCl does __________________________________ in water. o It does _____________________________________ into independent ions. Solubility Rules: A compound is __________________________ in a particular liquid if it dissolves in that liquid; a compound is __________________________ if it does not dissolve in the liquid. For _______________________________________, empirical rules of solubility have been deduced from observations of many compounds. Soluble Compounds Exceptions For example: Ca(NO3)2 is _______________________ Hg2Cl2 is _______________________ LiBr is _______________________ CaSO4 is _______________________ Insoluble Compounds Exceptions For example: Al2S3 is _______________________ K2S is _______________________ CaCO3 is _______________________ Na3PO4 is _______________________ Pb(OH)2 is _______________________ NH4OH is _______________________ Practice: Use the solubility rules to predict which of the following substances are likely to be soluble in water: Aluminum nitrate Magnesium chloride Rubidium sulfate Nickel (II) hydroxide Lead (II) sulfide Magnesium hydroxide Iron (III) phosphate Silver bromide