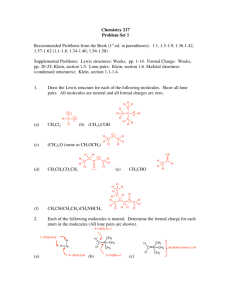
Chapter 10: Chemical Bonding

CHEMISTRY 100
CHAPTER 10: CHEMICAL BONDING
Homework problems: 29, 35, 37, 45, 49, 53, 55, 57, 63, 67, 71, 87, 89, 99, 101, 111,
116
Outline
I.
Lewis Structures
A.
Octet rule (rule of 8)
1.
for Representative elements (not transition or inner transition metals)
2.
2s, 6p electrons
3.
noble gas electron configuration
4.
"octet" for hydrogen and helium is 2 electrons (1s, but no 1p subshells)
5.
Transition metals = d shell area of periodic table > octet rule doesn't apply
B.
Atoms
1.
Ba: or Ba 2+
C.
Ionic Compounds
1.
D.
Covalent compounds p. 325 or...
1.
count up valence electrons
2.
add or subtract for charge
3.
figure out how many electrons needed to fill octets
4.
use steps 1 and 2 to determine how many electrons that you have
5.
subtract electrons you have (4) from electrons needed (3), result is electrons shared
6.
each shared electron pair equals one bond
7.
arrange all outer atoms around the central atom (usually least electronegative)
8.
put in shared pairs (bonds) – make sure everything has single bond before going to double bonds or triple bonds
9.
do not violate octet rules (no more than 4 bonds or lone pairs to any atom unless 3rd row or lower a. why? empty d shells to use
10. double check your electron count
Examples: HCO
2
-
CCl
4
CO
2
II.
Resonance
A.
More than one way to draw some structures
B.
Resonance
1. Move pi electrons only (lone pairs, double bonds, triple bonds. Leave
Examples: HCO
2
all single bonds intact.
N
2
O
III.
VSEPR Theory
NO
3
-
1
A.
Valence Shell Electron Pair Repulsion Theory
B.
Shapes
1.
count sets of electrons
2.
1 set is 1 lone pair, or 1 single bond, or 1 double bond, or 1 triple bond a.
2 atoms/lone pairs = linear b.
3 atoms/lone pairs = trigonal c.
4 atoms/lone pairs = tetrahedral
IV.
Electronegativity
A.
Measure of how badly something wants electrons
B.
Flourine, being the smallest atom that wants e-, is most electronegative
V.
Polar and Non-Polar Covalent Bonds
A. Polar vs Non-Polar
1. not always shared equally
2. if shared equally, non-polar
2. if not, then polar (from dipole
3. two ways to indicate polar bonds a. arrows b. δ+, δ-
O
H H
Chapter Objectives
Knowledge
Remember rules for one method or drawing Lewis structures for covalent compounds
Comprehension
Recognize that ionic bonds formed by electron transfer, then attraction between opposite charges (usually a metal and a nonmetal, but sometimes involve polyatomic ions)
Understand that covalent bonds are electron sharing
Understand that electrons are not always shared equally (polar vs. non-polar)
Understand how the octet rule applies to some elements and why it doesn’t work for transition and inner-transition metals (fill octets to generate ion charges)
Predict electronegativity trends across the periodic table
2
Application
Use the octet rule to predict charges on atoms in the periodic table (not transition and inner-transition metals)
Interpret whether a bond is ionic or covalent from the position on the periodic
Table (refer to metals and nonmetals).
Apply nomenclature rules to compounds to name them (mono, di, etc. for non-metals/ both the roman numeral and the –ic, -ous ending for metal compounds)
Given the name for a compound, determine the formula
Using Lewis structures and VSEPR theory, predict the shape of electron density around a central atom
Indicate polar covalent bonds by either of the methods talked about in class (arrow to more electronegative or δ+, δ-)
Analysis
Draw Lewis Structures for simple covalent molecules
Draw resonance structures
3