Organic Chemistry
advertisement

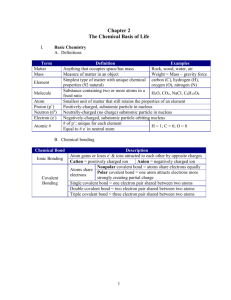
Organic Chemistry William H. Brown Christopher S. Foote Brent L. Iverson Organic Chemistry • The study of the compounds of carbon • Over 10 million compounds have been identified - about 1000 new ones are identified each day! • C is a small atom - forms single, double, and triple bonds - intermediate in electronegativity (2.5) - forms strong bonds with C, H, O, N, and metals Schematic View of an Atom • a small dense nucleus, diameter 10-14 - 10-15 m, which contains positively charged protons and most of the mass of the atom • an extra-nuclear space, diameter 10-10 m, which contains negatively charged electrons Electron Configuration of Atoms • The pairing of electron spins Electron Configuration of Atoms • Table 1.3 The Ground-State Electron Configuration of Elements 1-18 Polar and Nonpolar Covalent Bonds • Although all covalent bonds involve sharing of electrons, they differ widely in the degree of sharing • We divide covalent bonds into - nonpolar covalent bonds - polar covalent bonds Exceptions to the Octet Rule • Molecules containing atoms of Group 3A elements, particularly boron and aluminum :C l: : : : : :F : : :F: 6 electrons in the valence shells of boron and aluminum :C l B Boron trifluoride :C l: : : :F : Al Aluminum chloride Amines • contain an amino group; an sp3-hybridized nitrogen bonded to one, two, or three carbon atoms Methylamine (a 1° amine) C H3 N H C H3 Dimethylamine (a 2° amine) : H : : C H3 N H C H3 N C H3 C H3 Trimethylamine (a 3° amine) Shapes of a Set of 2p Atomic Orbitals • Three-dimensional shapes of 2p atomic orbitals