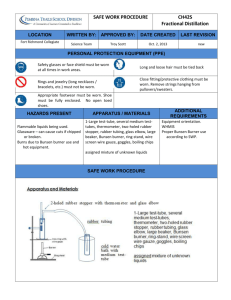
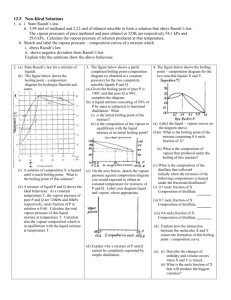
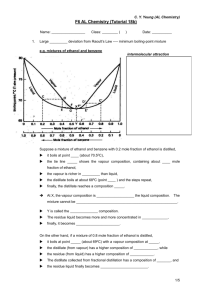
Exploration 1
advertisement

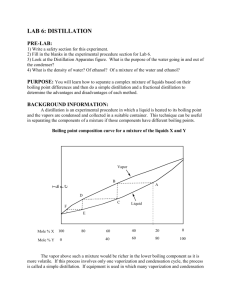
CHEM 211-2008 Experiment 2 Week 2 QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Gas Chromatogram How is the Boiling Point of a Mixture of 2 Liquids Related to the Boiling Points of the Pure Liquids? Quic kTime™ and a TIFF (Unc ompres sed) dec ompres sor are needed to see this pic ture. Abbe Refractometer A. Pre-lab Preparation Assignment: 1. Read: -This handout -Operation: 32: Refractive Index (Padías pp. 45-46 & 58-62) -Operation: 34: Gas Chromatography (Padías pp. 150-151 & 173-175) 2. Complete the Prelab Questions on the course website by 7:00 PM, Sunday, September 14. 3. Consider possible responses for the QOW (See below) 4. In your lab notebook: (See Lab Manual pp. 16-19 for format.) Treat Week 2 as a continuation of Experiment 2. Enter the Question of the Week after your notes from the Week 1 prelab discussion. 5. Bring your notebook and your ideas to the Monday Lab Discussion period. Record your Week 2 pre-lab discussion notes after the QOW for Week 2. B. Introduction: In Experiment 1 you explored the effects of changes in structure on the boiling point of a pure liquid. Last week, you extended this discussion to consider mixtures of liquids. You combined two liquids and observed the boiling point behavior of the mixture during a distillation. The result showed a significant effect of difference in the boiling points of the components of the mixture on the boiling point behavior over the course of the distillation. This week you will explore the composition of the distillate at different points in last week’s distillation. C. Question of the Day: How is the composition of distillate from a mixture of liquids related to the bp at which it was collected? Will the composition of the distillate collected from distillation of a mixture be the same as that of the original mixture or different? Will the composition of the distillate change as the bp at which it is collected changes? If the composition of the distillate is different from that of the original mixture, which of the original compounds would predominate? Would the identity of the predominant component change with changes in the bp at which the distillate is collected? CHEM 211 Experiment 2-WK-2 2 Boiling Points and composition of mixtures On Monday after discussing the results of the distillations in Experiment 2 – week 1, we will collect reasonable hypotheses about the QOW and explanations of the logic that led to each proposal. We will then have a brief discussion of the two new analytical methods, refractive index and gas chromatography, that you will use to analyze the composition of the distillate fractions you collected last week. In the lab you will do the analyses and determine if the compositions of fractions are related to their boiling points. D. Key Terms/concepts/techniques: Boiling point Refractive Index Gas Chromatography Percent Composition D. Safety Matters: a. Safety glasses are required. b. No sandals or open-toe shoes E. Waste Disposal: Non-halogenated organic liquids Halogenated organic liquids Category 4 Category 3