lab 6: distillation
advertisement
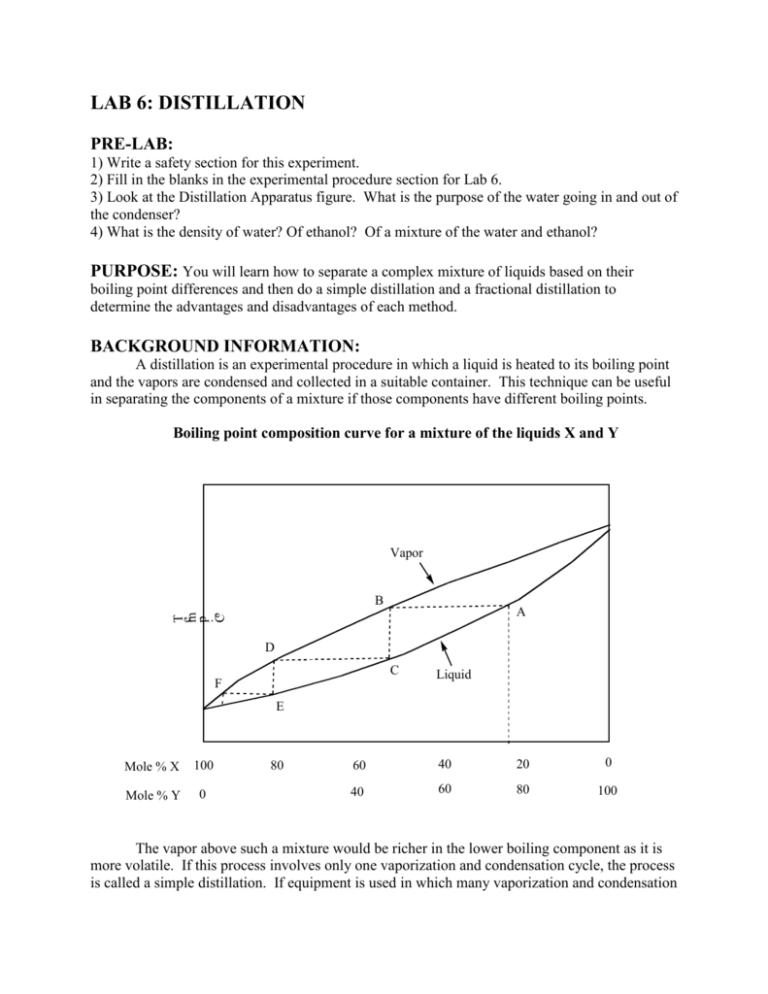
LAB 6: DISTILLATION PRE-LAB: 1) Write a safety section for this experiment. 2) Fill in the blanks in the experimental procedure section for Lab 6. 3) Look at the Distillation Apparatus figure. What is the purpose of the water going in and out of the condenser? 4) What is the density of water? Of ethanol? Of a mixture of the water and ethanol? PURPOSE: You will learn how to separate a complex mixture of liquids based on their boiling point differences and then do a simple distillation and a fractional distillation to determine the advantages and disadvantages of each method. BACKGROUND INFORMATION: A distillation is an experimental procedure in which a liquid is heated to its boiling point and the vapors are condensed and collected in a suitable container. This technique can be useful in separating the components of a mixture if those components have different boiling points. Boiling point composition curve for a mixture of the liquids X and Y Vapor B T em p . Co A D C F Liquid E Mole % X 100 Mole % Y 0 80 60 40 20 0 40 60 80 100 The vapor above such a mixture would be richer in the lower boiling component as it is more volatile. If this process involves only one vaporization and condensation cycle, the process is called a simple distillation. If equipment is used in which many vaporization and condensation cycles are achieved before the final liquid is collected, the process is called a fractional distillation. In today’s laboratory experiment you will do both types of distillation on a liquid mixture of ethanol and water. When a simple relationship exists between the liquid components of a mixture and the difference in boiling points is large, a simple distillation (see a diagram of this setup on the next page) often works well and will do a good job at separating the components. However, when the relationship is more complex or the difference in boiling points is not large, as in the case of ethanol - water, the separation is not as good. Fractional distillation can be used to better separate the components. Ethanol - water is more complex in that it forms an azeotropic mixture, that is, a mixture that forms a constant boiling mixture. The temperature of this azeotrope is lower than the boiling point of either component so it is impossible to obtain a 100 % pure sample in such cases. A fractional distillation of any mixture of ethanol - water will give at best a 95 % ethanol, 5 % water mixture. One would need to use other techniques to obtain 100 % ethanol (which is also called 200 proof ethanol). We will disregard this complication in today's experiment. There are other types of distillations that are useful in research environments and are beyond the scope of this course. A spinning band distillation is used if one needs to separate two liquids with similar boiling points. A vacuum distillation (one that is done under reduced pressure) is useful to distill high boiling components as many organic substances decompose near their atmospheric pressure boiling point. A steam distillation is useful when one wants to purify a high boiling material which is not soluble in water. In this case, since the vapor pressures of both liquids are separate, the material distills when the total of all vapor pressures equals the atmospheric pressure (which will always be below 100 degrees Celsius). Solid substances that have an appreciable vapor pressure can also undergo a similar process to distillation which is called sublimation. Since many organic liquids have a tendency to superheat (that is, achieve a temperature higher than the boiling point) before boiling action starts, it is very important to add a few boil easers (an inert solid material made from clay plates or anthracite coal) to the distillation flask before heat is applied. The boil easers give off a continual stream of bubbles that break the surface of the liquid and guarantee a smooth boiling process. Boil easers can only be used for one heating cycle and then lose their effectiveness. Fresh boil easers must be added if the liquid is reheated later. The thermometer placement to measure the temperature of the vapor is very important and often leads to confusion among students. The bulb of the thermometer must be placed just below the sidearm of the distilling head. This way, the entire mass of mercury in the bulb will be in the hot vapor and accurately measure the vapor temperature. Some confusion results when a student sees the liquid boiling and yet the thermometer reads room temperature. This is due to the fact that hot vapor has not reached the thermometer yet and the thermometer is not reading the temperature of the boiling liquid. An interesting question that you should answer and discuss in your report is: ‘Is the temperature of the boiling liquid the same as the temperature of the hot vapor?’ Simple Distillation Apparatus: Water Out Part B Water In Insert fractionating column here HEAT EXPERIMENTAL PROCEDURE: The instructor will assign each group either simple distillation or fractional distillation. Your report will need to include a discussion of both techniques so it is important to know the details of both. Question: At what point during the distillation should you measure the “boiling point” of the liquid? 1) Using a 50 ml round bottom flask and other equipment from the semi-micro glassware kit, do a distillation of 25 ml of a 50 % ethanol - 50 % water mixture. Be sure to use about 5 boiling chips in the flask and carefully place the thermometer so that accurate readings will be obtained. 2) Turn on the heat source. The boiling point of water is _________ ºC and the boiling point of ethanol is _________ ºC. The temperature should be set at _________ ºC. Question: What would happen if the temperature was set too high? Too low? 3) Collect about 10 ml of distillate into a graduate cylinder recording the temperature reading at 2 ml intervals. Do not collect more than 10 ml and be sure to accurately measure the exact volume you collected. DO NOT let the distillation flask become dry! Question: What are the physical characteristics of you the two compounds in the flask? 4) Weigh the graduated cylinder with the distillate in it and calculate the weight and density of distillate. Record your data in the CLASS DATA sheet provided. You will need all of the class data to complete your report - do not leave lab without this data! 5) Dispose of your waste and any left over distillate in the waste bottle in the hood. 6) Determine the approximate percent ethanol in the distillate. IMPORTANT INFORMATION ABOUT THE REPORT: A full lab report is required. Tabulate your data and discuss your result. Tabulate the class data. From the data you collected from class data discuss the effectiveness of each method. Graph the data in the table below and, using that graph and the density you obtained, show the percent of ethanol in your distillation condensate. WEIGHT % EtOH 0.0 10.0 20.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0 100.0 DENSITY (g/ml @ 20 degrees C) 1.000 0.982 0.969 0.954 0.935 0.914 0.891 0.860 0.838 0.818 0.780 Questions to consider: What characteristics of water and ethanol allow this distillation to work? In general which method gave the better separation? END OF EXPERIMENT. © 2010 Carberry and Carreon







