Document
advertisement

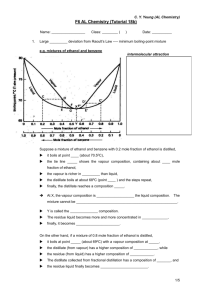
12.5 Non-Ideal Solutions 1. a. i. State Raoult’s law. ii. 3.94 mol of methanol and 2.22 mol of ethanol miscible to form a solution that obeys Raoult’s law. The vapour pressures of pure methanol and pure ethanol at 323K are respectively 54.1 kPa and 29.6 kPa. Calculate the vapour pressure of solution produced at that temperature. b. Sketch and label the vapour pressure – composition curves of a mixture which i. obeys Raoult’s law ii. shows negative deviation from Raoult’s law Explain why the solutions show the above behaviour. 2. (a) State Raoult’s law for a mixture of liquids. (b) The figure below shows the boiling point – composition diagram for hydrogen fluoride and water. 3. The figure below shows a partly completed boiling point-composition diagram (is obtained at a constant pressure) for the two completely miscible liquids P and Q. (a) Given the boiling point of pure P is 1000C and that pure Q is 900C, complete the diagram. (b) A liquid mixture consisting of 50% of P by mass is subjected to fractional distillation. What (i) is the initial boiling point of the mixture? (ii) is the composition of the vapour in equilibrium with the liquid mixture at its initial boiling point? 4. The figure below shows the boiling point – composition diagram for the two miscible liquids X and Y. (a) Label the liquid – vapour curves on the diagram above. (b)(i) What is the boiling point of the mixture containing 0.6 mole fraction of X? (ii) What is the composition of vapour that produced under the boiling of this mixture? (i) A solution of composition X is heated until it reach boiling point. What is the boiling point of this solution? (ii) A mixture of liquid P and Q shows the ideal behaviour. At a constant temperature T, the vapour pressure of pure P and Q are 120kPa and 40kPa respectively, mole fraction of P in solution is 0.60. Calculate the total vapour pressure of this liquid mixture at temperature T. Calculate also the vapour composition which is in equilibrium with the liquid mixture at temperature T. (c) On the axes below, sketch the vapour pressure against composition diagram you would expected to obtain at constant temperature for mixtures of P and Q. Label your diagram liquid and vapour, where appropriate. (c) What is the composition of the distillate that collected initially when the mixtures of the following compositions is heated under the fractional distillation? (i) 0.3 mole fraction of X Composition of distillate. (ii) 0.7 mole fraction of X Composition of distillate. (iii) 0.4 mole fraction of X Composition of distillate. (d) Explain how the interaction between the molecules X and Y causes the formation of this boiling point / composition curve. (d) Explain why a mixture of P and Q cannot be completely separated by simple distillation. (e) (i) Describe the changes of enthalpy and volume occurs when X and Y is mixed. (ii) What is the mole fraction of X that will produce the biggest variation? 5. The figure 1 shows the vapour pressure / molar composition curve for a mixture between propanone (CH3COCH3) and trichloromethane (CHCl3) at a constant temperature. (a)(i) Explain why the curve has the shape shown. (ii) What is the nature of interaction between the molecules of these two liquids? 6. i) By using the graph paper, draw the boiling point – composition diagram for the mixtures of liquid Q and R by following the given information below: The boiling point of pure Q = 780C The boiling point of pure R = 800C The boiling point of azeotrope = 680C Percentage of Q in azeotrope = 35% ii) In fractionally distilling a mixture consisting 70% of Q and 30% of R, explain the changes of temperature and composition of mixture which will be obtained through this distillation. [8] (b) Sketch the boiling point – molar composition curve for the mixtures of these two liquids. 7. The table below shows the partial vapour pressure of water and propan-1-ol in equilibrium with its liquid mixture as a function of this mixture composition at 250C. Mole fraction of water Partial vapour pressure / kPa Water Propan-1-ol 0.00 0.00 2.91 0.05 0.56 2.77 0.10 1.08 2.59 0.20 1.79 2.37 0.40 2.65 2.07 0.60 2.89 1.89 0.80 2.91 1.81 0.90 2.93 1.76 0.95 3.03 1.44 0.98 3.13 0.67 1.00 3.17 0.00 By using the data above, construct the vapour pressure - composition diagram for a mixture of water and propan-1-ol, thus determine (i) the areas where the liquid mixture shows an ideal behaviour. (ii) the vapour composition in equilibrium with the liquid mixture that containing 0.9 mole fraction of water. (iii) the vapour composition in equilibrium with the liquid mixture which containing 0.9 mole fraction of water if the mixture having an ideal behaviour for all of the composition. (c) How would you expect the temperature to change when equimolar quantities of these two liquids are mixed? Suggest a reason for your answer.