Fractional Distallation with SWP
advertisement

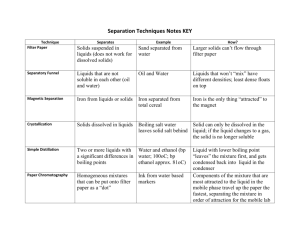
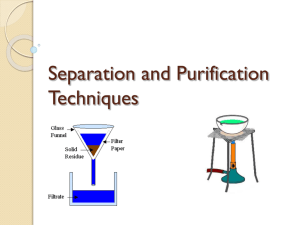
SAFE WORK PROCEDURE LOCATION Fort Richmond Collegiate CH42S Fractional Distillation WRITTEN BY: APPROVED BY: DATE CREATED Science Team Troy Scott Oct. 2, 2013 LAST REVISION new PERSONAL PROTECTION EQUIPMENT (PPE) Safety glasses or face shield must be worn at all times in work areas. Long and loose hair must be tied back Rings and jewelry (long necklaces / bracelets, etc.) must not be worn. Close fitting/protective clothing must be worn. Remove strings hanging from pullovers/sweaters. Appropriate footwear must be worn. Shoe must be fully enclosed. No open toed shoes. HAZARDS PRESENT Flammable liquids being used. Glassware – can cause cuts if chipped or broken. Burns due to Bunsen burner use and hot equipment. APPARATUS / MATERIALS 1-Large test-tube, several medium testtubes, thermometer, two-holed rubber stopper, rubber tubing, glass elbow, large beaker, Bunsen burner, ring-stand, wire screen wire gauze, goggles, boiling chips assigned mixture of unknown liquids SAFE WORK PROCEDURE ADDITIONAL REQUIREMENTS Equipment orientation. WHMIS Proper Bunsen Burner use according to SWP. Before you start: 1. Wear your goggles for the full duration of the lab. 2. Check all glassware and lab equipment to ensure there are no cracks or breaks. 3. When testing the liquids for flammability, carefully dip a small piece of paper in the liquid and light the liquid on the paper with a match. Do this over the sink, making sure that your work space is not cluttered. 4. Wipe the outside of your large test tube BEFORE lighting the Bunsen burner. If the liquid catches fire, turn off the gas first. 5. When heating your samples, carefully control the heat, making sure the sample does not overboil. 6. Turn off the Bunsen burner and stop heating your test tube if the sample boils dry. 7. Do not touch the ring-stand or glassware after heating, because it will be hot. Part A: 1. Obtain your materials and set up your apparatus as demonstrated. 2. Obtain the sample assigned to your group and right down all initial observations of the mixture. 3. Very carefully dip a small piece of paper in the liquid and try lighting the liquid on the paper with a match (while holding the paper over the sink). 4. Determine the density of a small amount of your sample. Record all values and show calculations. 5. Using a very small amount of sugar, see if a sugar will dissolve in the liquid. Part B: 1. Using a small volume (~ 5.0 mL) distill the sample. Use a single collecting tube and heat the liquid just enough to keep it boiling (but DO NOT over boil). Remember to use a few boiling chips in the test-tube. 2. Record the temperature of the VAPOR from the boiling liquid every 30 s while it distills. Continue to boil the liquid almost to dryness. 3. Before continuing, draw a graph of temperature vs. Time. (Using graph paper and showing all requirements for a proper graph.) Part C: 1. Fractionally distil about 25 mL of your sample. Be sure to label your test-tubes containing the fractions, so that you can keep track of them throughout the rest of the experiment. 2. Once your fractions have been collected, test each fraction for odour, flammability, and solubility of sugar. Recording all information in a neat and orderly fashion. 3. Determine the density of each fraction. Record. Part D: 1. Distil each fraction into a single test tube, recording the boiling temperature every 30 s until the fraction has nearly boiled away. Heat very slowly. 2. Draw a temperature vs. Time graph, showing the temperature vs. time data for each different fraction in a different colour on the same graph. REGULATORY REQUIREMENTS WS&H Act W210, Section 4, 5 Mb. Regulations 217/2006, Part 16, (Machines / Tools & Robots) Sections 16.1-16.18) Part 35, (WHMIS Application) Part 36, (Chemical & Biological Substances Application) CH42S Fractional Distillation Lab Purpose: a) to determine the composition of a mixture of liquids using the process of fractional distillation. b) To identify the substances in the mixture based on boiling point. Pre-lab Questions: 1. In what characteristic property must liquids differ before we can consider separating a mixture of them by fractional distillation? 2. The temperature-time graph shown below was made during the fractional distillation of a mixture of two liquids, E and F, and fractions were collected during the time intervals I, II, III, and IV. Liquid E has a higher boiling point than liquid F. What liquid or liquids were collected during each of the time intervals? 3. Briefly explain how the process of fractional distillation works at separating a mixture of liquids. Apparatus and Materials: See SWP above Procedure: See SWP above Questions (Part A & B: 1. What do you conclude from your graph about the number of fractions in your mixture? 2. At what temperatures should you shift from one collecting tube to another? Graph needs to be drawn and questions answered and checked before moving on to Part C of the lab. Instructor will sign your lab journal at this point. Data and Calculations: Report all data in a neat and organized table. Remember your units and follow rules for measuring and reporting significant figures. Label and show all calculations made. Any qualitative observations should also be in table form. Conclusion: Summarize your findings. Be sure to discuss the original solution and the fractions obtained. You should be able to research the identities of the fractions obtained. If identities are unsure, state what further tests could be done to help identify them. Sources of Error: State three sources of error and the effect they would have on the final results.