REVISION FOR ENERGY

ENERGY
DEFINITIONS
ENERGY
WORK
FORCE
The ability to perform work; usually measured in Joules (J)
Work (Nm or J) = Force (N) X Distance (m)
A push or pull that alters, or tends to alter, the state of motion of a body. Measured in Newtons (N)
POWER The rate at which work can be done ; or Work ÷ Time. Measured in
Watts (W)
EXAMPLE: If a 100m sprinter weighing 75kg moves 10 meters in 4 seconds, power will be calculated as follows:
KINETIC ENERGY
CHEMICAL ENERGY
Power = force (N) X distance (m)
Time (seconds)
= 750N
4s
= 187.5 W
Energy seen as muscle movement. E.g. running
Energy stored in compounds in our bodies. E.g. ATP,
Phosphocreatine, Carbs & Fats
POTENTIAL ENERGY Stored energy waiting to happen. E.g. ATP does nothing until the phosphate group is released with the help of
ATPase
ATP Adenosine Triphosphate – the only usable source of energy for work. ATP stored in the muscles provides kinetic energy for muscular contraction. Breaking down the high energy bond between the last two phosphate molecules acts as potential energy. The breakdown of ATP will only last 2-3 seconds.
High Energy Bonds
A P P P
ADP Adenosine Diphosphate
A P P
1
3)
2)
NOTE:
NOTE:
=
=
3)
2)
1)
ATP/PC or PHOSPHOCREATINE (PC) or ALACTIC SYSTEM
ATPase
1) ATP ADP +
Creatine kinase
P + Energy
PC
ADP +
P
P
+
+
C
Energy
+ Energy
ATP
=
Muscle Contraction
ATP is broken down into ADP and P and releasing Energy for Muscle
Contraction. This is an Exothermic Reaction , which releases energy.
When ATP levels fall and ADP levels increase, this stimulates the release of Creatine kinase which breaks down PC.
PC is broken down into P and C (by Creatine kinase) and releasing Energy to Re-synthesis ATP
ADP and P receive Energy from the breakdown of PC to join P onto
ADP to make ATP. This is an energy to be added.
2) and 3) are termed a
Endothermic Reaction
COUPLED REACTION reaction (Energy) is used in another reaction.
, which requires
due to the product of one
The ATP/PC System is the predominant energy system during high intensity, short duration movements or events, e.g. 100m sprint, long/triple jump, explosive jumping or diving etc.
TRAINING ADAPTATIONS
Anaerobic Training overloads the ATP/PC system and increases the body’s muscle stores of ATP and PC. This delays the threshold between the ATP/PC and the Lactic Acid
System andtherefore increases the potential duration of high intensity exercise for up to 1-
2 seconds.
2
KEY
GPH
PFK
LDH
NOTE:
ADVANTAGES AND DISADVANTAGES OF THE ATP/PC SYSTEM
ADVANTAGES
No O2 required
DISADVANTAGES
Only a small amount of ATP and PC stored in muscle cells
PC stored in muscle cell as readily available energy source
Very quick re-synthesis of ATP
Only one ATP is re-synthesised for one
PC
Only provide energy to re-synthesise
ATP for a maximum of 10 seconds
Provides energy for high intensity exercise
No harmful by-products that will cause fatigue
Recovery for this system is very quick due to quick re-synthesis of PC
NOTE: The ATP/PC System works under Anaerobic conditions.
The fuel for this system is Phosphocreatine.
This reaction takes place in the sarcoplasm of the muscle cells.
LACTIC ACID SYSTEM
This is also know as Anaerobic Glycolysis (the partial incomplete breakdown of Glucose into Pyruvic Acid)
GLYCOGEN (stored in the Muscles/Liver)
GPH
GLUCOSE (C
6
H
12
O
6
)
PFK 2ATP re-synthesised
LDH
PYRUVIC ACID (C
3
H
4
O
3
) LACTIC
ACID
(C
3
H
6
O
3
)
=
=
Glycogen Phosphorylase. Converts Glycogen to Glucose
Phosphofructokinase. Enzyme that helps break Glucose down into Pyruvic Acid
= Lactate Dehydrogenase. Converts Pyruvic Acid into Lactic Acid
There is NO Oxygen present!!!
3
NOTE: The main limitation of this system is the Onset of Blood Lactate
Accumulation (OBLA) – which lowers the pH and inhibits enzymes.
NOTE: The Lactic Acid system is the predominant energy system for the 400m sprint and for midfield games players that have lots of high intensity sprints with no time for recovery
TRAINING ADAPTATIONS
Repeated bouts of anaerobic training which overload the LA system also increase the body’s tolerance to lactic acid. This will increase the body’s stores of Glycogen. This also delays the effect of OBLA and prolongs the Lactic Acid threshold.
ADVANTAGES AND DISADVANTAGES OF THE LACTIC ACID SYSTEM
ADVANTAGES
Relatively large amount of Glycogen stored in muscles/liver and is readily available
Re-synthesises two molecules of ATP – more than ATP/PC System
DISADVANTAGES
Not as quick as the ATP/PC System
Produces Lactic Acid, which is a fatiguing by-product
Requires few chemical reactions than
Aerobic System, so provides a quicker supply of energy
GPH and PFK enzyme activation due to
Reduces pH of muscle cell (making it more acidic) which inhibits the enzyme action
Stimulates pain receptors a decrease in PC
Can work aerobically and anaerobically Net effect is muscle fatigue and pain
Provides energy for high intensity exercise lasting between 10 and 180 seconds
NOTE: The Lactic Acid System works under Anaerobic conditions.
The fuel for this system is Carbohydrates (in the form of Glycogen).
The reactions take place in the sarcoplasm of the muscle cells.
4
AEROBIC SYSTEM
1)
GLYCOGEN
GPH
GLUCOSE
PFK
COENZYME A +
OXALOACETIC ACID +
PYRUVIC ACID
ACETYLE CoA
CITRIC ACID
2) OXALOACETIC ACID
CO2
KREBS
CYCLE
3)
O2
+ H
NAD AND FAD
NADH and FADH
H2O
H
H+ e ‾
ELECTRON
TRANSPORT
CHAIN
2ATP
Sarcoplasm
Mitochondria
(matrix)
Mitochondria
(cristae)
2 ATP
34 ATP
5
1)
2)
= Aerobic Glycolysis.
Same reactions as Lactic Acid System, apart from
Pyruvic Acid combines with coenzyme A to form Acetyl CoA.
= Krebs Cycle.
The Acetyl CoA combines with oxaloacetic acid to form Citric
Acid. Citric acid is then taken into the Krebs Cycle where:
CO2 is produces and removed via the lungs
Hydrogen atoms are removed (oxidation)
Energy is produced to re-synthesis 2 molecules of ATP
Oxaloacetic acid is regenerated
3) = Electron Transport Chain.
The Hydrogen atoms (from Krebs Cycle) combine with the coenzymes NAD and FAD to form NADH and FADH.
These are then carried down the Electron Transport Chain where hydrogen is split into H+ an d e‾. This takes place in the cristae of the mitochondria where three important events take place:
The hydrogen electron (e‾) splits from the hydrogen atom and passes down the ETC
This provides sufficient energy to re-synthesise 34 ATP molecules
The hydrogen ion (H+) combines with oxygen to form water (H2O)
The equation for the Aerobic System would be:
C
6
H
12
O
6
+ 6O
2
= 6CO
2
+ 6H
2
O + Energy to re-synthesise 38 ATP
FATS
Triglycerides (fats) are broken down by enzymes termed lipases into free fatty acids
(FFA) and glycerol and used as an energy fuel within the aerobic system. FFA are broken down into Acetyl CoA, which enters and is broken down by the Krebs Cycle and the ETC in the process termed beta-oxidation .
FFA produce more Acetyl CoA and consequently produce far greater energy than the breakdown of glycogen/glucose. However, FFA’s require 15% more O2 than that required to break down glucose. Therefore, glycogen and glucose are the preferred energy fuel during moderate or high intensity activity.
TRAINING EFFECTS
Aerobic training causes a number of beneficial adaptations which help to improve the aerobic energy system’s efficiency to re-synthesise ATP:
Increased storage of muscle and liver glycogen
Increased metabolism of aerobic enzymes
Earlier use of FFA’s as a fuel thereby helping to conserve glycogen stores
The net effect of the above adaptations is that they increase/prolong the aerobic threshold thereby increasing the potential intensity of performance. This delays muscle fatigue by increasing the intensity at which the onset of blood lactate accumulation is reached and by maximising its efficiency to remove lactate during periods of recovery.
6
DEFINITIONS
ANAEROBIC
ANAEROBIC
GLYCOLYSIS
ATP
ATPase
COUPLED
REACTION
CRISTAE
ELECTRONS
ADVANTAGES AND DISADVANTAGES OF THE AEROBIC SYSTEM
ADVANTAGES DISADVANTAGES
Large potential glycogen and Free Fatty
Acids (FFA) stores available as an efficient energy fuel
Efficient ATP re-synthesis when good O2 supply guarantees breakdown of FFA
Slower rate of ATP re-synthesis compared with LA system
Requires more O2 supply (15% more for
FFA)
More complex series of reactions Large ATP re-synthesis. 38 ATP from one molecule of glucose, compared to 2 from LA system and 1 from ATP/PC system
Provides energy for low/moderate intensity, high duration exercise (3 minutes to 1 hour)
Cannot re-synthesise ATP at the start of exercise due to initial delay of O2 from the cardiovascular system
No fatiguing by-products. CO2 and H2O are easily removed
NOTE:
Limited energy for ATP during high intensity, short duration work
The Aerobic System works under Aerobic conditions.
The fuel for this system is Glycogen or Fat as well as requiring Oxygen to function .
The reactions take place in the sarcoplasm of the muscle cells, Matrix of the Mitochondria and Cristae of the Mitochondria .
CONTROLLING ENZYMES
Energy System
ATP/PC System
Lactic Acid system
Aerobic System
Controlling Enzymes
Creatine kinase
Phosphofructokinase
Phosphofructokinase
Activator
Increase in ADP
Decrease in PC
Increase in adrenalin/decrease in insulin levels
A reaction that can occur without the presence of oxygen
The process of breaking down glucose into Pyruvic Acid
The only immediately usable source of energy in our bodies
The enzyme that helps break down ATP to release energy
The products of one reaction are then used in another reaction (see
ATP/PC System)
Internal membrane/compartments/fold-like structures within the mitochondria
A negatively charged particl e of an atom (e‾)
7
ELECTRON The process of combining H+, e‾ and O2 to produce water and
TRANSFER CHAIN provide energy for the re-synthesis of 34 ATP
ENDOTHERMIC A chemical reaction that requires energy to be added for it to progress
EXOTHERMIC
KREB’S CYCLE
MATRIX
A chemical reaction that releases energy as it progresses
The cyclical process of breaking down pyruvic acid in CO2, H+ a nd e‾ whilst providing the energy for the re-synthesis of 2 ATP
PYRUVIC ACID
SARCOPLASM
Intracellular fluid within the mitochondria where oxidation takes place
MITOCHONDRIA The ‘Powerhouse’ of the cell where all aerobic processes take place
Product of the partial breakdown glucose during anaerobic glycolysis
The gel-like content of the muscle that contains all the organelles of the cell. It also stores glycogen, fat, proteins, enzymes and myoglobin
THRESHOLD
The point when one energy system stops being the predominant energy provider for the re-synthesis of ATP
8
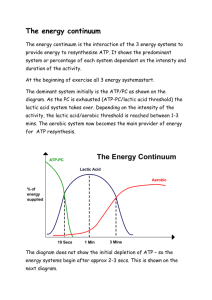
ENERGY CONTINUUM
This is ‘ the relative contribution of each energy system to ATP re-synthesis determined by the intensity and duration of exercise .’
In any sporting situation, energy is provided by all three energy systems, and the contribution of each is determined by the intensity and the duration of the exercise. Some activities/sports are mainly aerobic while others are anaerobic. Energy systems rarely work in isolation.
100
Capacity of
Energy
System (%) Aerobic System
Lactic Acid System
0
10
ATP/PC System
20
Time
2min 5min+
Graph showing Energy System Interaction Linked to Exercise Duration
Identify the predominant energy system used in the following types of exercise:
Activity % ATP/PC % Lactic Acid % Aerobic
100m Sprinter
Shot Putter
Marathon Runner
800m Runner
Tennis Player
Netball Centre
Football
Goalkeeper
Badminton
200m Swimmer
9
FACTORS AFFECTING THE ENERGY SYSTEM USED
A combination of exercise intensity and duration can determine the predominant energy system(s) being used. When exercise intensity is anaerobic (high intensity, short duration), then the ATP/PC and LA Systems will be predominant. If the exercise intensity is aerobic (medium/low intensity, long duration), then the Aerobic
System will be predominant.
When the aerobic system cannot supply energy quick enough, it has to use the LA system to continue to provide energy for re-synthesis of ATP. During high intensities lactate production will start to accumulate above resting levels. This is termed
Lactate Threshold.
When blood lactate levels reach 4mmol/L (normal resting levels are 1-2mmol/L), the exercise intensity is referred to as
‘the Onset of
Blood Lactate Accumulation’ (OBLA).
OBLA continues to increase if exercise intensity is maintained or increased and will cause muscle fatigue.
After training the intensity level for lactate threshold is increased and this will delay the point at which OBLA is reached and therefore increases the potential duration/threshold of the LA energy System.
You will need to be able to explain the main factors that affect the energy system utilise:
Exercise Intensity and Duration
(above)
Energy System Threshold
O2 Transport/Supply
Food/Fuel available
Enzyme Activation Levels
Fitness Level
THRESHOLDS
The Threshold for any system is ‘ the point at which that energy system is unable to provide energy.
’ Or ‘the point at which one energy system is taken over by another as the predominant energy system to provide energy for ATP resynthesis.’
Energy System Thresholds
Performance Duration Practical Example
Less than 10 seconds
10-90 seconds
90 secs – 3 mins
3+ mins
Energy System(s)
Involved (predominant in bold)
ATP/PC
ATP/PC
LA
LA
Aerobic
Aerobic
Triple Jump/100m sprint
200-400m sprint
100m swim
Boxing (3 min rounds)
800/1500m
Low impact aerobics class
Marathon
10
The energy system threshold alters in response to a combination of both intensity and duration of exercise and will not always go through each energy system in turn. For example, a cyclist cycling at a low intensity will be using the Aerobic System, although when going up hill they may exceed the intensity threshold of the aerobic system and the lactic acid system will take over as the predominant energy system. In team games, players will switch between the three energy systems.
OXYGEN AVALABILITY
If there is O2 present then the aerobic system can provide energy to re-synthesise ATP. If
O2 supplies falls below that demanded by the exercise then the aerobic system threshold is met and the lactic acid system will start to break down glucose anaerobically.
ENZYME ACTIVATION
Factors Affecting Enzyme Activation for the Energy Systems
Activating Factor Activating Energy System Releases Controlling
Enzyme(s)
Creatine Kinase PC Increase in ADP; decrease in ATP
Decrease in PC PFK
PFK
LA System
Aerobic System Increase in adrenalin; decrease in insulin
FITNESS LEVEL
The more aerobically fit the performer, the more efficient their cardiovascular and respiratory systems are. Aerobic athletes have also shown that they can start to use FFA’s earlier during sub-maximal exercise, which conserves glycogen stores. The overall effect is that the aerobic threshold in terms of intensity and duration can be increased as the lactate threshold/OBLA would be delayed.
A typical untrained athlete would reach OBLA at about 50-56% of their VO2 max, whereas an aerobic-trained athlete would not reach OBLA until about 85-90% of their VO2 max.
An anaerobic-trained athlete will increase their ATP/PC, glycogen stores, anaerobic enzymes and tolerance to lactic acid. All of this would increase the threshold of both
ATP/PC and Lactic Acid Systems.
FUEL AVAILABILITY
If the body has sufficient stores of PC, it is able to use the ATP/PC system for very high intensity, short duration activity/movements. PC stores are limited, but are available at the start and after recovery during exercise. If exercise starts too high then PC stores will quickly deplete and exercise at that intensity cannot be sustained. PC stores can be conserved by pacing and re-synthesising PC stores during recovery periods using spare energy from the aerobic system.
11
Glycogen is the major fuel for the first 20 minutes of exercise. This is due to O2 supplies being limited as it takes 2-3 minutes for the cardiovascular system to supply sufficient O2.
As well as glycogen being readily available in the muscles, requires less O2 and is easier to break down tha n FFA’s. About
After about 20-45 minutes there is a greater breakdown of fats alongside glycogen as the energy fuel. FFA’s are a more efficient fuel than glycogen, but require 15% more O2. If a performer has larger muscle/liver glycogen store, then they can perform work aerobically at a higher intensity.
Glycogen stores become nearly depleted after about two hours, then FFA’s have to be used for aerobic energy production, and unless exercise intensity is reduced it can bring on the sudden onset of fatigue (‘hitting the wall’). Once OBLA is reached the body has insufficient O2 available to burn FFA’s and will then have to break down glycogen
‘anaerobically’ to re-synthesise ATP.
12
EXAM QUESTIONS
JANUARY 2002
1 During physical activity such as a physical education or games lesson, an athlete
2 will use a combination of energy systems.
The graph in Fig. 1 shows the relative contribution of aerobic and anaerobic energy metabolism during maximal physical effort of various durations.
100
X Y Aerobic Metabolism
80 Duration of Maximal Exercise
Secs Minutes
% of Total 10 30 60 2 4 10 30 60 120
Energy
Yield
%
Anaerobic 90 80 70 50 35 15 5 2 1
60 %
Aerobic 10 20 30 50 65 85 95 98 99
40
20
Anaerobic Metabolism
0
0 10 20 30 40 50
Maximal Work Time (mins) a)
Fig. 1
With reference to Fig. 1, provide the missing information A , B and C from the table that summarises the predominant energy system being used at points X and Y on the graph. (3 marks)
Fuel Used Active Enzyme
At X
At Y
Predominant
Energy System
ATP-PC
Aerobic
PC
Glycogen and
Fats
Creatine Kinase
A
Site(s) of
Reaction
B
C c) Explain how ATP is created aerobically when you are performing a continuous exercise or a named sport. Compare the relative efficiency of this ATP production with anaerobic routes. (10 marks)
13
JUNE 2002
No Questions.
JANUARY 2003
1 In many activities in Physical Education and Sport, performers will use all three energy systems and a range of energy fuels. a) Fig. 1 show the relationship of the energy systems utilised over a one-mile race by a top class performer.
B
100
%
90
Intensity 80
A
Energy System Y
Aerobic of
Process 70
60
50
40
30
Resting 20
Level
10
0
ATP/PC
0 1 2 3 4
Time (mins) i)
Fig. 1
Identify energy system Y and outline the physiological processes occurring in the body between points A and B on the graph
(3 marks)
14
c)
% Energy
Fig. 2 shows that exercise intensity and exercise time determine the type of fuel utilised for energy creation.
100 from Fat/
Carbohydrates
80
60
Carbohydrates
40
20 Fats
0
0 20
% Fat/
70
65
60
Carbohydrate 55
40 60 80
Exercise Intensity (% VO2 max)
100
Fats
`
Metabolism 50
45
40
35
30
Carbohydrates
0 20 40 60 80 100
Exercise Time (min)
Fig. 2
Using Fig. 2, explain how intensity and duration of exercise play such an important role in the type of food fuel we use. (6 marks)
2
JUNE 2003
1
No Questions.
JANUARY 2004
A cool down helps to return the body to its resting state by oxidising lactic acid and lowering heart rate. b) i) Describe the energy system that causes a build up of lactic acid in the body. (5 marks) c) Performers may use the ATP/PC system during short, sharp explosive movements. Outline the advantages and disadvantages of this energy system. (7 marks)
15
JUNE 2004
1 During a competitive team game a performer will use a combination of the three energy systems.
(i) Name an energy system. Identify the missing information A, B, C and D in the table below for your chosen energy system.
(4 marks) a)
Site of Reaction Fuel Used Active Enzyme Molecules of ATP
Produced
2
2 c) c)
1
JUNE 2005 b) b)
A B
(ii) What is meant by the term Energy Continuum? For the energy system you selected in (i), identify a situation in a team game when this system would be predominant. Explain your answer.
(5 marks)
JANUARY 2005
1 a) Define the terms energy, work and power, giving the units of measurements for each. (3 marks)
ATP is a most important compound.
C D
Explain why ATP plays such a major role during physical activity.
(3 marks)
A trained athlete can perform at a higher percentage of their VO2 max before reaching OBLA than an untrained person.
(i) Explain OBLA.
The body uses oxygen during the recovery from exercise resulting in an elevated rate of aerobic respiration. The first stage of this process involves the breakdown of glycogen to pyruvic acid. of glycogen.
(3 marks)
Describe the remaining stages that use oxygen to complete the breakdown
(8 marks)
Performers usually rely on all three energy systems for ATP resynthesis.
However, at any one time one energy system may be predominant.
Sketch a graph to show how the predominant energy system depends on duration of exercise. (4 marks) c) Two tests designed to evaluate the strength in the rectus abdominis muscle are Maximum number of sit ups in 30 seconds the Abdominal Curl Sit Up Test .
and Time until exhaustion in
Identify the type of Strength being evaluated and the Energy System being used in each of the tests. (6 marks)
16
2
JANUARY 2006
No Questions.
JUNE 2006
1 a) Fig. 1 shows the changes in ATP and PC during a 100m sprint
Sprint Exercise
% of
Resting
100 x
80
60
Value
40
20
0
0 x
2 x
PC
4 x
6 x
8
Time (s)
ATP x
10 x
12 x
Exhaustion
14
Fig. 1
(i) The table below describes the predominant energy systems being used in the 100m sprint.
Identify the missing information X and Y. (2 marks)
Type of Reaction
Anaerobic
Fuel Used
PC
Site of Reaction
X
Controlling
Enzyme
Y c)
(ii) Using the graph above, explain the relationship between ATP and
PC levels during the 100m sprint.
During a match, a games player will work at different intensities and produce energy from both aerobic and anaerobic pathways. This will affect the energy system and the fuel used. For example, when a Basketball player slam-dunks the ball into the basket, they are using the ATP/PC system and the chemical fuel, Phosphocreatine.
(4 marks)
Using examples from a sport of your choice, explain when and why a performer uses the Lactic Acid and the Aerobic energy systems and fuels during a competitive match.
Discuss the effects of level of aerobic fitness, availability of oxygen and food fuels on the efficiency of the aerobic energy system. (13 marks)
17
JANUARY 2007 b) If the workload was increased during the interval training session, the performer would reach onset of blood lactate accumulation (OBLA).
(i) Define OBLA and describe its effect on the skeletal muscle.
(3 marks)
Explain the principle of a coupled reaction using the ATP/PC system as your example. (4 marks) c)
JUNE 2007
2 c) Fig.2 represents the energy systems used by high-level performers in their specialist events. a) 100m sprinter
ATP/PC
Lactic Acid
Aerobic b) Marathon runner c) Football goalkeeper
Sketch a similar model to show the energy systems being used by team player, other than a goalkeeper, in a team game of your choice. Using examples from the game situation, explain when and why your performer uses each of the three energy systems. (8 marks)
18
JANUARY 2008
1 a) Knowledge of the three energy systems underpins exercise and sport physiology.
(i)
Energy System
Name an energy system and identify the missing information A, B and C for this system.
__________________________________
(3 marks)
FUEL USED SITE OF REACTION CONTROLLING ENZYME
A B C
(ii) Sketch a graph of energy supplied against time to show when each of the three energy systems is predominant in relation to duration of exercise. (3 marks)
JUNE 2008
2 c) Swimmers often rely heavily on the use of the lactic acid system for ATP resynthesis.
1
Describe the lactic acid system and discuss the advantages and disadvantages of using this system. (8 marks)
JANUARY 2009 b (ii) An average time for completion of an agility test is 17 seconds for males and 19 seconds for females.
Identify the two predominant energy systems that would be used by an average performer during the completion of this test.
Discuss the advantages and disadvantages of one of the energy systems you have identified. (6 marks)
19