Infrared Spectroscopy: Teacher's Notes & Functional Groups
advertisement
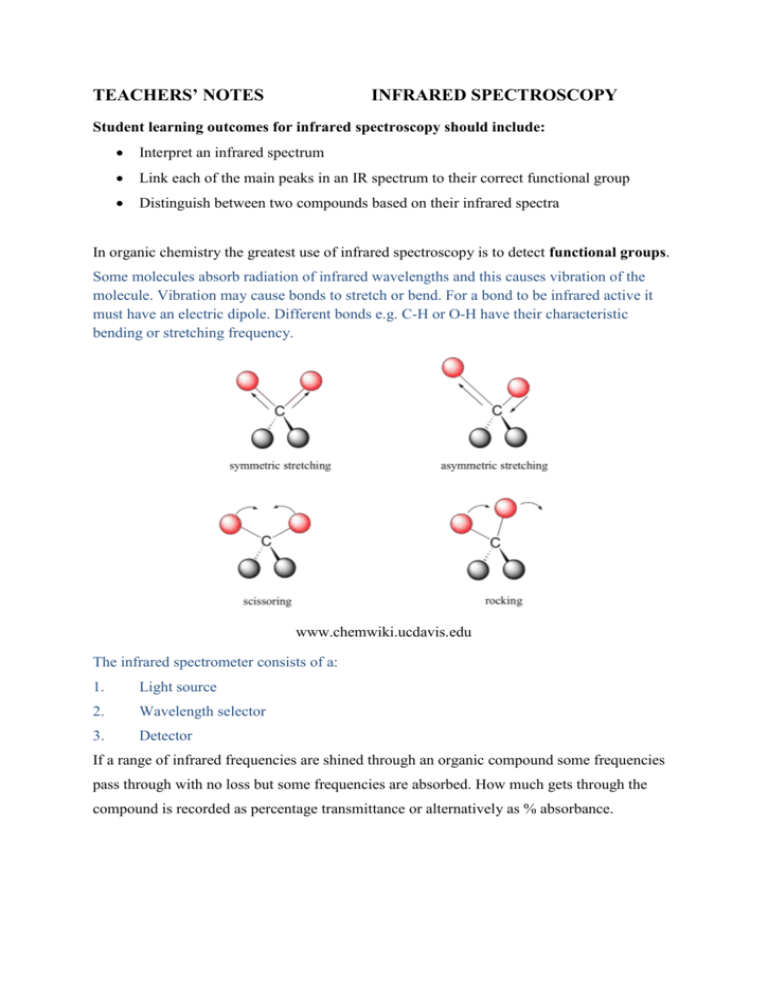
TEACHERS’ NOTES INFRARED SPECTROSCOPY Student learning outcomes for infrared spectroscopy should include: Interpret an infrared spectrum Link each of the main peaks in an IR spectrum to their correct functional group Distinguish between two compounds based on their infrared spectra In organic chemistry the greatest use of infrared spectroscopy is to detect functional groups. Some molecules absorb radiation of infrared wavelengths and this causes vibration of the molecule. Vibration may cause bonds to stretch or bend. For a bond to be infrared active it must have an electric dipole. Different bonds e.g. C-H or O-H have their characteristic bending or stretching frequency. www.chemwiki.ucdavis.edu The infrared spectrometer consists of a: 1. Light source 2. Wavelength selector 3. Detector If a range of infrared frequencies are shined through an organic compound some frequencies pass through with no loss but some frequencies are absorbed. How much gets through the compound is recorded as percentage transmittance or alternatively as % absorbance. The following correlation chart shows the characteristic absorptions of different functional groups. Bond Types of compound C-H O-H Alkanes, Alkenes, Aldehydes Alcohol Carboxylic acid Amine Alcohols, carboxylic acids. esters Aldehydes, ketones, carboxylic acids, esters Alkenes Chloroalkane Bromoalkane Iodoalkane N-H C-O C=O C=C C-Cl C-Br C-I Range/ cm-1 3100-2800 3700-3300 3600-2500 broad 3500-3100 sharp 1250-1050 1850-1600 1500-1600 600-800 500-600 500 An example of an infrared spectrum for butanoic acid is seen below: A carboxylic acid has the following bonds: Carbon-oxygen double bond C=O Carbon-oxygen single bond C-O Oxygen- hydrogen O-H Carbon- hydrogen C-H Carbon – carbon single bond C-C The C-C and C-O bond absorption is found in the fingerprint region where there are lots of peaks so it is difficult to assign these. The C=O bond is found around 1700cm-1, so this is useful as is the O-H at 2500-3300cm-1, which is very broad and the C-H at 2800-2900cm-1. The fingerprint region is found on the right hand side of an infrared spectrum from about 1500-500 cm-1. It can be used to compare two similar compounds. For example propan-1-ol and propan-2-ol. Both would show absorption around 3300-3700 cm-1 due to the –OH group but their fingerprint regions would show different peaks. www.chemguide.co.uk www.chem-ilp.net/labTechniques/IRVideo.htm 1. The above website has a 2 minute video on how to prepare a sample for IR spectroscopy. Linked here is also an animation of the IR radiation passing through a sample and an example of an IR absorption chart. www.vicsco.com.au/downloads.Infrared.pdf 2. The above website has brief notes on: How infrared spectroscopy works including a diagram of the various parts Applications of infrared spectroscopy Analysis of an IR spectrum www.bluffton.edu/~bergerd/classes/cem222/infrared/oxygen.html 3. The above website has various examples of infrared spectra for molecules containing carbon-oxygen bonds (an alcohol, aldehyde, ketone, carboxylic acid, ester). Good to compare the IR spectra of each compound. Has stick models of the molecules next to each IR as well. http://www.youtube.com/watch?v=DDTIJgIh86E&feature=relmfu 4. A 6 minute 30 second video on the introduction to infrared spectroscopy and how an infrared spectrum is produced. www.youtube.com/watch?v=0nxrFd7i_fM 5. Figure it out (The IR spectrum song) 4 minutes 50 seconds. Goes through the various regions on the IR spectrum and relates it to the functional groups.