Infrared Spectroscopy KEY
advertisement
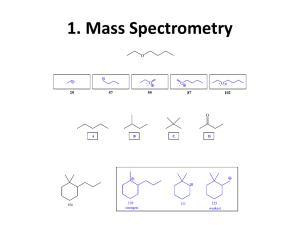
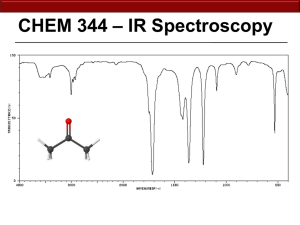
SI WS #3 – Infrared Spectroscopy 1/22/12 1. Label the wavelength and amplitude of this wave: (Wavelength – length from one wave maximum/minimum to the next maximum/minimum) (Amplitude – height of a wave measured from midpoint to a peak) (Frequency – number of waves passing a point in a certain amount of time) 2. Fill in each of the blanks with either “directly” or “inversely.” Directly proportional means that as one factor increases, the other factor also increases. Inversely proportional means that as one factor increases, the other factor decreases. Think about the equations that relate wavelength, frequency, and energy. a. Wavelength and frequency are _________________ proportional. (inversely) b. Energy and frequency are ___________________ proportional. (directly) c. Energy and wavelength are __________________ proportional. (inversely) 3. What functional groups are likely present if they give IR readings at the following frequencies/wavenumbers? Use Table 13.1 on p. 439 of your textbook. a. 1735 cm-1 (ester) b. 2100 cm-1 (alkyne) c. 700 cm-1 (alkyl chloride) d. 1540 cm-1 (nitro group) e. 1720 cm-1 and 2500-3100 cm-1 (carboxylic acid) 4. Using IR spectroscopy, how could you tell these two isomers apart? (Isomers are compounds with the same molecular formula, but different structural formulas.) a. Acetone (C=O bond) and b. Propanoic Acid (C=O bond, -OH bond) Prop-2-en-1-ol (-OH bond, C=C bond) and Propanal SI WS #3 – Infrared Spectroscopy 1/22/12 5. Where would the following compounds show IR absorptions? a. (-OH bond, CN bond, aromatic bonds) b. (Br bond, C=O bond, alkene, alkane) 6. What type of compound is this? (Amine – propylamine) SI WS #3 – Infrared Spectroscopy 1/22/12 7. What type of compound is this? (carboxylic acid – butanoic acid)