Topics to be covered1998
advertisement

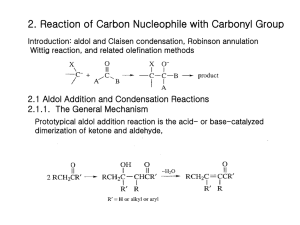
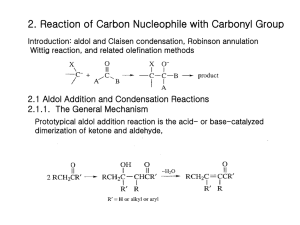
T. V. RajanBabu Chemistry, 730 Autumn 1998 Overview of topics to be covered Introduction Expectations for the course Challenges in organic chemistry Efficiency and selectivity (chemo-, regio-, and stereo-) in organic synthesis Stereochemical Principles Constitution, configuration and conformation Specifying stereochemistry - CIP notation, Fischer convention Chirality as a property of matter Enantiomeric relationships Enantiomeric excess (ee), optical activity - how to determine it Origin of chirality - asymmetric carbon atom, asymmetric heteroatom with lone-pairs, chiral molecules without asymmetric centers - how to specify configuration in axially chiral molecules Diastereomeric relationships- definitions, specifying diastereomers Threo and erythro vs syn and anti (threo/erythro R. I. P.) Consequences of diastereomerism- resolution of enantiomers and limitations- ananlytical methods based on diastereomerism for the determination of ee’s: GC, HPLC, X-ray, NMR Diastereoselective synthesis: e. g., Evan’s oxazolidinones for alkylation (more later) Kinetic resolution - principles, stoichiometric, catalytic - organometallic and enzymatic, with examples Asymmetric catalysis - diastereomerism in transient intermediates - principles, examples - hydrogenation Stereochemistry of dynamic processes Stereoselective and stereospecific reactions (definitions, examples, common mistakes) Racemization process Prochiral relationships (Prochiral groups - pro-R, pro-S groups and how to identify them) Prochiral faces (re and si, how to identify them) Enantioselective enzymatic processes, how to use them in synthesis, Asymmetric synthesis: Stoichiometric and catalytic use of reagents 1 Examples of the latter: Sharpless epoxidation, Rh-catalyzed hydrogenation, ketone reductions; Itsuno-Corey reductions, Ru-catalyzed hydrogenations ( for illustration only, details to be covered in next course) Diastereotopic relationships - pro-R, pro-S Enantiotopic vs diastereotopic groups - how to distinguish between them - in naming, by NMR Diastereotopic faces - consequences for stereoselective synthesis - Cram’s rule ( no details yet!) Conformational, Steric and Stereoelectronic Effects Conformation of ethane, Pitzer strain Conformation of butane and van der Waals and gauche interactions Conformations of CH3XHn, ethers, amines, alcohols Conformations of terminal alkenes, carbonyl compounds (aldehydes and ketones) Conformations of dienes, enals and enones Conformations of esters Conformations of amides - Relationship between K and free energy difference between Z and E amides - How to calculate K from Go ( also see later under A values) Conformational analysis of cyclic compounds - cyclohexanes, chair, boat, twist conformations - proof Substituted cyclohexanes - ‘A’ values of various groups - how to determine ‘A’ values Conformational effects on reactivity - oxidation of axial vs equatorial alcohols, hydrolysis of axial vs equatorial esters haloketone conformations allylic strain, cyclohexenes six-membered heterocycles 4, 5, 7, 8 -membered cycloalkanes Curtin-Hammett principle: mathematical derivation and examples (conformational or diastereomeric equilibria); examples of Rh-catalyzed hydrogenation of acetamidocinnamates amine-oxide pyrolysis Conformation of decalins and perhydrophenanthrenes Strain in rings - 3-11 membered rings, propallanes, Bredt’s rule, effect on reactivity Tetrahedrane, [1.1.1]-propallane, Cubane,dodecahedrane and C60 synthesis and reactivity of Bredt olefins 2 Anomeric effect in sugars and in other compounds, difference between- and - glycosides- oxidation, reaction with ozone, radical generation Other stereoelectronic effects: SN2 reaction, E2-elimination, Fürst-Plattner rule with examples from steroids Reactions of isomeric 1,2-bromohydrins from cyclohexenes (elimination vs ring contraction etc.) SN2’ reactions Ring expansions, fragmentation reaction of 1,2-hydroxytosylates Ring contractions, Fragmentation reactions - stereochemistry, reaction conditions Solvolytic fragmentation reactions Reductive cyclopropane opening - applications Tortional starin and stereoelectronic effects -Reduction of cyclic ketones, solvolysis of cyclic tosylates, Axial vs equatorial approach to cyclic carbonyl compounds by nucleophiles Klein / Cieplak models Tortional interactions in bicyclic systems Ring closure and ring size (Baldwin’s rules) - enthalpy and entropy of activation Bürgi-Dunitz angle, Radical cyclization reactions under kinetic vs thermodynamic conditions as an illustration of limits of Baldwin’s rules, also symmetry controlled reactions Kinetic vs thermodynamic control, Hammond postulate, use of energy level diagrams - examples Nucleophilic addition to carbonyl compounds Cram’s rule and variations - Original Cram’s rule, the chelate model, Cornforth modification and the Felkin-Anh model - Where these are applicable, where not - examples. Carbon acids, enolates and enamines: alkylation Acidity and basicity of organic compounds Kinetic and thermodynamic acidities Generation and use of alkyl lithiums, lithium amides Structures of alkyl lithiums, amides Carbanions stabilized by other functional groups Malontes, acetoacetates, nitrocompounds etc. 3 Enolates - kinetic vs thermodynamic - regiochemistry in unsymmetrical ketone enolates - how to prepare regiochemically pure enolates Other carbanions in synthesis - dithianes and corresponding sulfoxides, nitrocompound, cyanoalkanes Acidites of phosphonium and sulfonium compounds and ylides (for chemistry see later) Enols, enamines and metalloenamines in synthesis Mechanism of acid and base catalyzed enolization, kinetic vs thermodynamic control Detailed mechanism of -substitution of a carbonyl compound (e. g., bromination) Carbanions as nucleophiles Enolate structure - X-ray structures of enolates, effect of aggregation, how to control reactivity C vs O-alkylation: Solvent effects, HMPA, counter ion effects, effect of alkylating agents Hard and soft acid-base principle Generation and regioselective alkylation of enolates: silyl enol ethers, enolacetates, Li /amine reductions, cuprate additions, -in bicyclic systems- stereochemistry Alkylation of malonates : synthesizing substituted acetic caids Alkylation of acetoacetates: synthesizing acetone derivatives Alkylation of aldehydes, esters, amides and nitriles Generation and reactions of dianions: How to substitute at the more basic -position Generation and alkylation of chiral enolates - making optically active -substituted acid and alcohol derivatives (Evans’ auxiliaries) Lewis-acid mediated alkylation of silyl enol ethers (Mukaiyama reaction) - especially for SN1 -active substrates Nitrogen analogs of enols and enolates - enamines and metallaenamines Synthesis of enamines Reactivity, alkylation, alkylating -position of aldehydes Making optically pure -alkylated ketones (Meyers’ auxiliary) Alkylation of hydrazone anions (A1,3- strain, models), Enders’ N-amino-2-methoxymethylpyrrolidine Hydrazone cuprates - acetaldehyde anion equivalent Application of enamines - acylation: synthesis of 1,5-dicarbonyl compounds, stereoelectronic effects Michael reaction - stabilized enolates- malonates, acetoacetates, nitronates Michael reaction of kinetic enolates -special acceptors 4 Use of ketene silyl acetals as surrogates of enolates Ketene silyl acetals in Lewis acid, fluoride, thermal additions to enones Michael reactions with enamines Robinson annulation Umpolung - dithianes and analogs, cyanohydrin anions - (Acyl anion synthons) Metallated enol ethers including cuprates Nagata cyanation Reactions of carbon nucleophiles with carbonyl compounds Aldol condensation - acid/base catalyzed, mechanism, differences Directed aldol - how? Enolate regiochemistry, stereochemistry Examples of directed aldols (Scheme 2.3) - Li, Si, B, Zn Mukaiyama conditions Definitions of syn/anti, lk, ul Zimmerman-Traxler transition states- dependence of size of substituents Enolate generation (Z and E): Ireland model Examples of substituent, solvent, counter ion, base effects on enolate stereochemistry Boron enolates, including limitations Break down of Z-T transition states: two cases (explain with the aid of models): Aldol reactions of silyl enol ethers with Lewis acids Use of TAS+ Me3SiF2– in aldol reactions, counter ion effects Generation and use of other metal enolates: Zr, Si, Ti, Mg (-> E), Ti (–> E), Ti (–>Z), Sn (–>Z) Enantiomerically pure aldols Masamune, Evans auxiliaries and models Chelated and unchelated transition state models to make all four aldol stereoisomers using diffrent metals Asymmetric catalysis in aldol synthesis : Mukaiyama, Carreira, Evans and others Ito’s gold-catalyzed aldol reactions (a Knoevenagel-type reaction, see later) of -isocyanoacetates Aldol reactions of ester enolates (how to make E- enolates better) 5 Carbanions from esters, acids, amides and nitriles (CS B, Scheme 2.4) Intramolecular aldols including Robinson annulation (see earlier) The Hojos and Parrish reaction Mannich reaction Knoevenagel condensation Acylation of nucleophilic carbon by esters (Claisen condensation: inter- and intra- molecular) Reactive acylating agents: Anhydrides, acid chlorides, ethoxymagnesium malonates, N-acylimidazoles, methoxymagnesium carbonate, ethyl cyanoformate Acylation of enamines, equivalent for enolate acylation Acylation of -ketosulfoxides Wittig and Related reactions Stabilized vs unstabilized- mechanism and stereochemistry: making Z and E- olefins Salt- free Wittig reagents, Schlosser modification Synthetic applications (scheme CS B 2.12, 2.13) Stabilized ylides Wardsworth-Emmons modification and alternatives Limitations Horner-Wittig reaction Silicon equivalent of Wittig reaction, Peterson olefination; How to make Z and E olefins Sulfonium and sulfoxonium ylides (differences, applications) Tebbe and related reactions; carbene complexes of transition metals Functional group interconversions Alcohols to alkylating agents Mitsunobu reaction Mechanistic considerations of SN2 reactions Useful SN2 reactions for C-heteroatom bond formation Phase transfer catalysis Alkylation of amines and amides Oxygen nucleophiles, protection/deprotection of alcohols Making esters, epoxides 6 S, Se, N, P nucleophiles Activation of carboxylic acids, macrolactonization 7