word document
advertisement

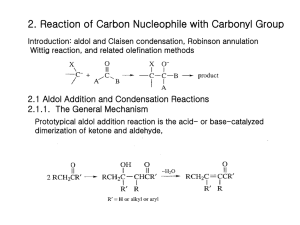
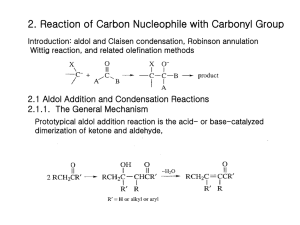
Chem 110b–Section 2 Exam 2 Review Sheet Chapter 16 Physical properties of aldehydes (CHO) and ketones relative to alkanes and alcohols (dipole moment, bp, H-bonding ability, solubility in H2O); nomenclature; spectroscopic identification of CHO/ketones from other carbonyls and each other; why are -hydrogens so acidic?; enols and enolates; keto-enol tautomerization: when is the enol favored? the keto form favored? Preparation of CHO: oxidation of 1° ROH; ozonolysis; reduction of acyl chlorides (LiAlH(OtBu)3) and esters (DIBAL-H) with mechanisms; addition to nitriles. Preparation of ketones: ozonolysis, Friedel-Crafts acylation, oxidation of 2° ROH; addition of cuprates to acyl chlorides, hydration of alkynes (only methyl ketones); addition to nitriles. Nucleophilic addition to carbonyls: Burgi-Dunitz trajectory, tetrahedral intermediate, product dictated by the nature of the substituents on the carbonyl and the nature of the nucleophile. You should know the conditions required, the products formed and the mechanism for the transformation of CHO and ketones into of each of the following species: hydrates, hemiacetals, acetals, thioacetals, cyanohydrins, imines, enamines, and alkenes (via the Wittig reaction). In addition, where are some places we see these groups in nature? Why can an acetal “protect” a carbonyl from nucleophilic attack? What type of amine leads to imine formation? to enamine formation? Why don’t 3° amines add to carbonyls in appreciable amounts? It should be noted that imines and enamines are nitrogen analogs of carbonyls and enols, respectively. Chapter 17 Acidity of -hydrogens; enolization results in racemization (1 stereocenter) or epimerization (molecule has multiple stereocenters); haloform reaction (products, mechanism). Aldol reaction: mechanism of aqueous acid and base catalyze addition and dehydration; aldol reactions are reversible under these conditions prior to dehydration; thermodynamic driving force is dehydration (-hydroxycarbonyls are not the isolated products). Crossed aldol reactions in aqueous acid or base: only useful if one partner has no -hydrogens, partner with -hydrogens is added slowly to the other partner and acid or base (why?); ClaisenSchmidt reaction=crossed aldol between an aldehyde and a ketone; intramolecular examples of crossed aldols to form rings. Reformansky reaction (products can be -hydroxycarbonyls) Thermodynamic vs. kinetic enolates; lithium diisopropylamide (LDA) forms kinetic enolates irreversibility; using LDA, crossed aldols can be performed when both partners have hydrogens (how is this carried out?); alkylation of lithium enolates; Evans oxazolidinone uses steric bias as a means of controlling the enantioselectivity of alkylation. Conjugate addition to ,-unsaturated carbonyls: cyanide, cuprates, amines and enolates (Michael additions) are nucleophiles; coupling of conjugate addition to aldol condensation to make rings (Robinson annulation).