Enolates Structure-HSAB # 35
advertisement

Chemistry 730, Au 2005
Carbanions as Nucleophiles (C&S A, p. 432-439; 290-298)
Structure of enolates:
• ambident anion
• An enolate be alkylated either at C or O
Reactivity can be explained using Hard and Soft Acid and Base (HSAB) principle
(CSA p. 292-293); CSA p. 22
R. G. Pearson, J. Chem. Ed. 1968, 45, 581, 643.; Chem. Rev. 1975, 75, 1.; J. Am. Chem. Soc. 1967, 89,
1827.
• “Reactions occur best between species that are matched in hardness or softness”
e.g., Hard nucleophiles prefer hard electrophiles and soft nucleophiles prefer soft electrophiles.
Definitions:
Soft base (or nucleophile): Donor atom of high polarizability, low electronegativity, easily oxidized, has
empty low-lying orbitals
Hard Base (nucleophile): Donor atom of low polarizability, high electronegativity, difficult to oxidize,
has empty orbitals of high energy.
Soft acid (electrophile): Acceptor of low positive charge, large size, has several easily excited outer
electrons
Hard Acid (electrophile): Acceptor of high positive charge, small size, does not have easily excited
electrons.
Examples
1
Chemistry 730, Au 2005
Bases (nucleophiles)
Soft: RSH, RS–, I–, R3P, CN–, CO, Olefins, ambident enolate carbanion
Borderline: Br–, N3–
Hard: H2O, OH–, ROH, RO–, RCO2–, F–, Cl–, NO3–, NH3, RNH2, ambident enolate oxyanion
Acids (electrophiles)
Soft: I2, Br2, RCH2X, RSX, RSeX, Cu(I), Ag(I), Pd(II), Pt(II)
Borderline: Cu(II), Zn(II), Sn(II), R3C+, R3B
Hard: H–X, H+, Li+, Na+, K+, Mg2+, Ca2+, Sn(IV), Ti(IV), R3SiX
Examples
(a) Nucleophilic substitution versus elimination in alkyl halides
(b) C vs O Alkylation of enolates
2
Chemistry 730, Au 2005
(c) C vs O Alkylation and leaving group ability
Important:
• Hard-hard combination is driven by coloumbic attraction, usually through has early transition state
leading to O-alkylation (not much covalent bond formation)
• Soft-soft combination has late TS, partial bond formation is important leading to C-alkylation
• Consider covalent bond energies in this context: energetically C-alkylation is better, other things
being equal:
{C=O + C–C ([–173 + (–)81] = –254 kcal/mol)} is better than {C–O + C=C ([–79 + (–)145] = –
224 kcal/mol)}
Another interesting effect is alpha effect: (p.
, CS A)
e. g., HOO– is more nucleohilic than HO– and
H2NNH2 and HONH2 mor enucleophilic than NH3 (WHY ?)
Medium effects in alkylation of enolates: Dielectric constants
3
Chemistry 730, Au 2005
DMSO - 47; DMF - 37; NMP - 32; HMPT -30
• Polar medium facilitates breaking up of aggregates
• Nucleophilicity of anions depends on degree of solvation
• Protic hydrogen-bonding solvents tend to solvate anions, especially hard nucleophiles: therefore soft
nucleophiles are more nucleophilic in such media.
• Best nucleophilic substitutions proceed in polar aprotic solvents. They tend to coordinate to the cation
, increasing the reactivity of the anion.
• Maximum rate for enolate alkylation is seen in polar medium; Li+, Na+, K+ are all solvated under
these conditions.
In a given solvent order of cationic reactivity is:
BrMg+ < Li+< Na+<K+<NR4+
13
C chemical shifts confirm the difference of electronegativity of the carbanions in various solvents.
• Electron density of a given carbanion increases in the following order for various solvents:
• THF/HMPA. DME>THF>Ether (House, J. Org. Chem. 1976, 41, 1209.)
• For various metal ions the order is: K+ > Na+. >Li+
EXPLAIN:
4
Chemistry 730, Au 2005
• Also the trends seen in Table p. 438, CS A C vs O alkylation of K-salt of ethyl acetoacetate as a
function of the leaving group)
Summary
• To maximize O-alkylation use highly polar aprotic solvent (HMPA/THF, DMF, NMP) , with a hard
leaving group (tosylate or mesylate).
• To maximize C-alkylation, use aprotic solvent of low dielectric constant and dipole moment (THF,
DME) with soft leaving group (e. g., a halide)
• Solvents like THF and DME are extensively used: low bp, easy to remove after reaction, enhanced
reactivity upon mixing with HMPA or TMEDA or crown-ethers
• To maximize O-alkylation use dissociting counte r ions: R4N, K+, Na+ etc.
• Crown ethers also enhance reactivity of nucleophiles:
• 12-crown-4 (small cavity) - for sequestering Li
• 18-crown-6 (large cavity) - for sequestering K and Na
READ: X-Ray structures of enolates (unsolvated vs solvated (p. 436, CS B)
LOOK UP THE X-RAY STRUCTURES OF ENOLATES
5
Chemistry 730, Au 2005
Generation and alkylation of aldehydes esters amides and nitriles
• Ketones most common
• But equaly viable for other realtively acidic compounds except aldehydes (see for this substrate work
with the imines)
OVERHEAD SCHEME 1. 8
6
Chemistry 730, Au 2005
Alkylation of Dianions (CS B, p. 19 and Handout)
HANDOUT - READ, READ, CAREFULLY WORKOUT THE EXAMPLES!!!
7
Chemistry 730, Au 2005
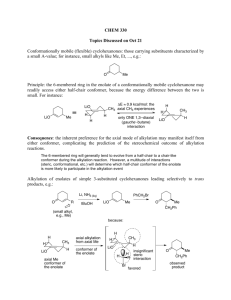
Diastereoselective synthesis: Alkylation of chiral enolates (Evans enolates)
Lookup the synthesis of the starting oxazolidinones (JACS 1982, 104, 1737)
HANDOUT - READ, CAREFULLY WORKOUT THE EXAMPLES!!
8