bifunctional chemistry
advertisement

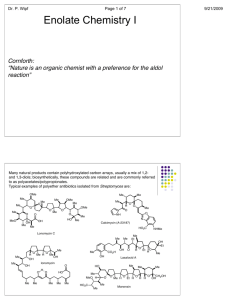
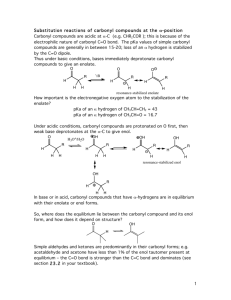
BIFUNCTIONAL CHEMISTRY (06522) Contents Overview Keto-enol tautomerisation Reactions of enols: C-Hal and C-N=O bond formation Reactions of enols and enolates with aldehydes and ketones Reactions of enolates with esters and acid chlorides Reactions of enolates with alkylating agents Conjugate addition reactions and the Michael reaction Reactions of enol / enolate-like molecules such as enamines, silyl enol ethers and nitronates Learning outcomes: At the end of the course you will be able to: 1. Keto-enol tautomerisation Demonstrate the mechanisms of both acid and base-catalysis of tautomerisation and draw tautomeric form(s) of a given molecule. Discuss factors controlling tautomeric stability. 2. Enols and enolates: C-Hal and C=N-OH bond formation Work out the products arising from the reaction of enols or enolates with halogens and the nitrosonium (NO+) ion. 3. Enols and enolates: the aldol, Claisen and Dieckmann reactions Work out the product(s) arising from the reaction of enols or enolates with aldehydes, ketones and esters. 4. Crossed condensation reactions Work out the product(s) arising from the reaction of an enolate from one carbonyl compound with a different carbonyl compound, identifying factors which give rise to combinations producing product(s) with either high or low selectivity. 5. Lithium enolates and enolates of 1,3-dicarbonyl compounds Work out the product(s) arising from the reaction of lithium enolates with alkyl halides. Demonstrate the use of stable enolates from β-keto esters and 1,3-diesters for alkylation reactions, with a subsequent decarboxylation process. 6. Conjugate addition reactions Work out the product(s) arising from the conjugate addition reactions of stable enolates with α,β-unsaturated carbonyl compounds. 7. Enamines and silyl enol ethers Compare the reactions of simple enamines and enol ethers with those of enols and enolates. 8. Nitroalkanes and cyanoalkanes Compare the reactions of the anions of nitroalkane and cyanoalkanes with those of enolates. Suggested reading Organic Chemistry, J. Clayden, N. Greeves, S. Warren and P. Wothers, Oxford University Press. Chapters 21, 26, 27, 28, 10, 29 (1st Edition) or Chapters 20, 22, 25, 26 and in part 17 and 28 (2nd Edition). Background / revision: Chapters 6, 12, 14 (reactions of aldehydes, ketones and esters – lectures from year 1) (1st Edition) or Chapters 6, 10 and 11 (2nd ed.). Also recommended is Chemistry Of The Carbonyl Group, A Programmed Approach To Organic Reaction Mechanisms, S. Warren (Wiley, 1974) Bifunctional Compounds, RS Ward, OP (Oxford Primer No. 17): useful in parts Similar chapters will be found in all Organic Chemistry texts, e.g. T. Solomons & C. Fryhle, F. Carey, Maitland Jones etc. See http://www.hull.ac.uk/php/chsanb/teaching.html for a set of these notes and past exam paper questions with answers. Work book problems must be completed as the course progresses. Dr AN Boa, C120b, a.n.boa@hull.ac.uk Page 1 Page 2 Page 3 Page 4 Page 5 Page 6 Page 7 Page 8 Page 9 Page 10 Page 11 Page 12 Page 13 Page 14 Page 15 Page 16 Page 17 Page 18 Page 19 Page 20 Page 21 Page 22 Page 23 Page 24 Page 25 Page 26 Page 27 Page 28 Page 29 Page 30 Page 31 Page 32 Page 33 Page 34 Problems Lecture 1: Draw all possible enols of the following compounds. Lecture 2: Explain the following observations. The optical rotation [α]D of compound A goes from -96° to 0° after treatment with sodium methoxide, and the optical rotation of B changes from +66° before to +45° after recrystallization from acetic acid. Lecture 3: Work out the product(s) from the following cyclisations. Lecture 4: Work out the product(s) from the following condensations. Page 35 Lecture 5: Work out the product from the following sequence. Lecture 6: Work out the product from the following sequence. Lecture 7: Work out the product from the following reactions. Page 36 SUMMARY OF KEY REACTIONS Page 37