Carbon Chemistry Test
advertisement

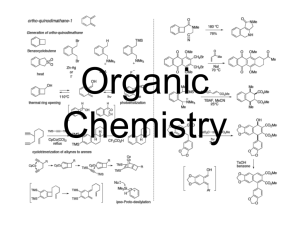
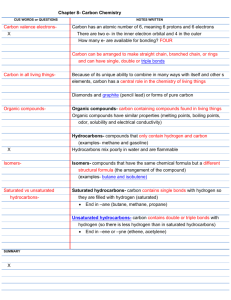
Carbon Chemistry Test 1. Carbon is able to bond with other element in many different ways because it has a. six protons b. four electron c. six valence electrons d. four valence electrons 2. Which form of pure carbon is so hard that it can be used in cutting tools? a. diamond b. graphite c. hydrocarbon d. fullerenes 3. What is another name for carbon compounds? a. carbohydrates b. organic compounds c. fullerenes d. organic compounds 4. The element whose atoms can make straight chains, branched chains and rings is a. carbon b. hydrogen c. nitrogen d. oxygen 5. Which form of pure carbon is formed of layers that slide past one another? a. diamond b. fullerene c. graphite d. isomer 6. What can you tell about methane (CH4) from its molecular formula? a. It contains four carbon atoms. b. It contains one hydrogen atoms. c. It contains four hydrogen atoms. d. It forms groups of four molecules. 7. Which compounds have the same molecular formula but different structures? a. hydrocarbons b. isomers c. organic compounds d. polymers 8. Substances that provide the energy and raw materials the human body needs are a. nutrients b. substituted hydrocarbons c. esters d. unsaturated hydrocarbons 9. Which of these compounds has the maximum possible number of hydrogen atoms on its carbon chain? a. hydrocarbon with double bonds b. hydrocarbon with triple bonds c. saturated hydrocarbon d. unsaturated hydrocarbon 10. Compounds that contain only the elements carbon and hydrogen are called a. carbon chains b. hydrocarbons c. isomers d. organic compounds 11. What shapes do hydrocarbons NOT form? a. straight chains b. branched chains c. ring-shaped chains d. geodesic domes 12. What is the shape of pure carbon fullerenes? a. branched chain b. hollow ball with a pattern like a geodesic dome c. flat layers d. hard, solid crystal shaped like a ball 13. How many chemical bonds can each carbon atom form? a. one b. two c. three d. four 14. A very large organic molecule made up of chain of smaller molecules is called a a. monomer b. polymer c. saturated hydrocarbon d. substituted hydrocarbon 15. The classes of polymers found in all living things are a. vitamins and minerals b. halogen compounds, alcohols, organic acids, and esters c. simple carbohydrates and complex carbohydrates d. carbohydrates, lipids, proteins, and nucleic acids 16. A carbohydrate is made up of the elements carbon, hydrogen plus a. oxygen b. oxygen and nitrogen c. oxygen and sulfur d. oxygen and potassium 17. A large, complex molecule built from smaller molecules joined together is a(n) a. composite b. alloy c. monomer d. polymer 18. If a polymer is compared to a long train of identical train cars, each car represents a a. alloy b. chemical bond c. composite d. monomer