Supplemental Assignment #3 Organic Chemistry guided notes
advertisement

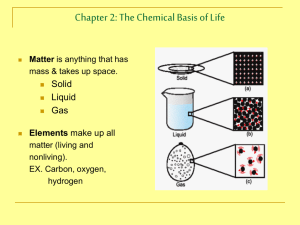
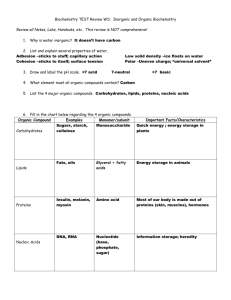
HB - ORGANIC CHEMISTRY NOTES Chapter 2 Organic compounds contain ______ and hydrogen and often other elements like oxygen. Carbon is the backbone because it has four _________electrons which allows it to make four __________bonds. These bonds are formed by two atoms sharing electrons and can be single bonds, _____________bonds or even __________bonds. A _____________ is an organic compound that contains only the elements carbon and hydrogen. If all the bonds are single, and the molecule has the maximum possible amount of hydrogen, then the compound is called a _______________ hydrocarbon. These are the alkane compounds which have names ending with ane. An unsaturated hydrocarbon may have either double or triple bonds or both. These compounds known as alkenes and alkynes have names that end with __________ or _______________. Carbon molecules can be straight _________, branched chains or even rings. Compounds that have the same chemical formula, but different structural formulas are called _______________. Substituted hydrocarbons are compounds in which one or more hydrogen has been replaced by an atom or group of atoms. These are called ___________ groups. Some common functional groups include: -OH (hydroxyl group) which forms an ______________. Organic acids include a carboxyl group which looks like: Amines have a: –NH2 group and esters form when an organic acid reacts with an __________. Most organic compounds have names reflecting the number of carbon atoms involved. These names use prefixes for numbers: 1 6 2 7 3 8 4 9 5 10