Chemistry of Life: The Chemical Compounds in Cells
advertisement

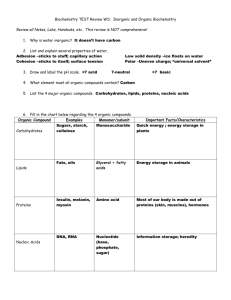
Chemistry of Life: The Chemical Compounds in Cells Quiz Date: ___________ Study Sheet Know this information. 1. An element is any substance that cannot be broken down into simpler substances. 2. There are 92 naturally occurring elements and more than 20 that have been created by scientists in labs. 3. The most common elements in living things are carbon, hydrogen, oxygen, and nitrogen (CHON). 4. The Periodic Table of Elements is a chart that shows all of the elements known. The table is organized to show their atomic numbers, how elements are related, their atomic weights, their abbreviations and other information. 5. Each element has an abbreviation called a symbol. (You should know the symbols of some of the common elements such as oxygen = O, carbon = C, hydrogen = H.) 6. The smallest unit of an element is called an atom. An element is made up of only one kind of atom. The word atom comes from the Greek word, atomos, which means indivisible. 7. Atoms consist of three types of subatomic particles: protons, neutrons, and electrons. 8. Protons are found in the nucleus and have a positive charge. Neutrons are also found in the nucleus and have no charge. Electrons are found in orbits called shells surrounding the nucleus. Electrons have a negative charge. 9. The atomic number of an element is the number of protons or electrons it has. 10. The atomic mass (weight) of an element is the total of the protons and neutrons in the nucleus. 11. The behavior of electrons makes it possible for atoms of elements or atoms of the same element to come together to form compounds. Electrons between atoms can be shared or captured. This sharing or capturing called bonding keeps the atoms together. 12. When two ore more different elements combine by sharing or capturing electrons they form a compound. Water is an example of a compound. 13. The smallest unit of a compound is a molecule. H2O is the formula for a molecule of water. This means a molecule of water consist of 2 atoms of hydrogen and 1 atom of oxygen. (You should be familiar with other common compounds such as CO2 (carbon dioxide) and NaCl (salt). 14. Many of the compounds found in living things contain the element carbon, which is usually combined with other elements. 15. Most compounds that contain carbon are called organic compounds. Compounds that do not contain carbon are called inorganic compounds. (The exception to this definition is carbon dioxide. It contains carbon but is not an organic compound. Water and salt are also inorganic compounds.) 16. The most important groups of organic compounds found in living things are: a. carbohydrates b. lipids c. proteins d. nucleic acids 16. These organic compounds are found in the food we eat. The food we eat comes from living things (plants and animal products). 17. Carbohydrates are organic compounds made of the elements carbon, hydrogen, and oxygen. Sugars and starches are examples of carbohydrates. Carbohydrates supply energy. Fruits, vegetables, and grains are carbohydrates. When you eat carbohydrates your body breaks them down into simple sugars which provide energy. 18. Proteins are large organic molecules made up of carbon, hydrogen, oxygen, nitrogen, and, in some cases, sulfur. Foods that are high in protein are meats, eggs, fish, nuts, and beans. Proteins are used by the body for repair and growth. Proteins make up the organelles in cells. Proteins are made up of smaller molecules called amino acids. Twenty different amino acids make up a large variety of the proteins used by living things. 19. An enzyme is a type of protein that helps make chemical reactions take place in a living thing. For example, the enzymes in your saliva speed up the digestion of food by breaking down starches into sugars in your mouth. 20. Lipids (fats) are energy rich organic compounds made of carbon, hydrogen, and oxygen. Lipids contain even more energy than carbohydrates. Cells store the energy in lipids for later use. 21. Cholesterol is a lipid. Cholesterol is an important ingredient in making cell membranes. The liver produces the cholesterol needed to make cell membranes. Excess cholesterol in foods can damage arteries. 22. Nucleic acids are very large organic molecules made of carbon, oxygen, hydrogen, nitrogen, and phosphorus. Nucleic acids contain the instructions that cells need to carry out all of life’s activities. 23. There are two kinds of nucleic acids, deoxyribonucleic acid and ribonucleic acid. These two nucleic acids are known as DNA and RNA. 24. Water makes up about two-thirds of your body. Water is vital to the functioning of your body. Most chemical reactions that take place in cells can only occur when the substances are dissolved in water. Without water most chemical reactions within cells could not take place. 25. Water also helps cells keep their shape and size. Water also keeps the temperature of cells from changing too fast. .