1-Paracresol is used as a disinfectant and in the manufacture of
advertisement
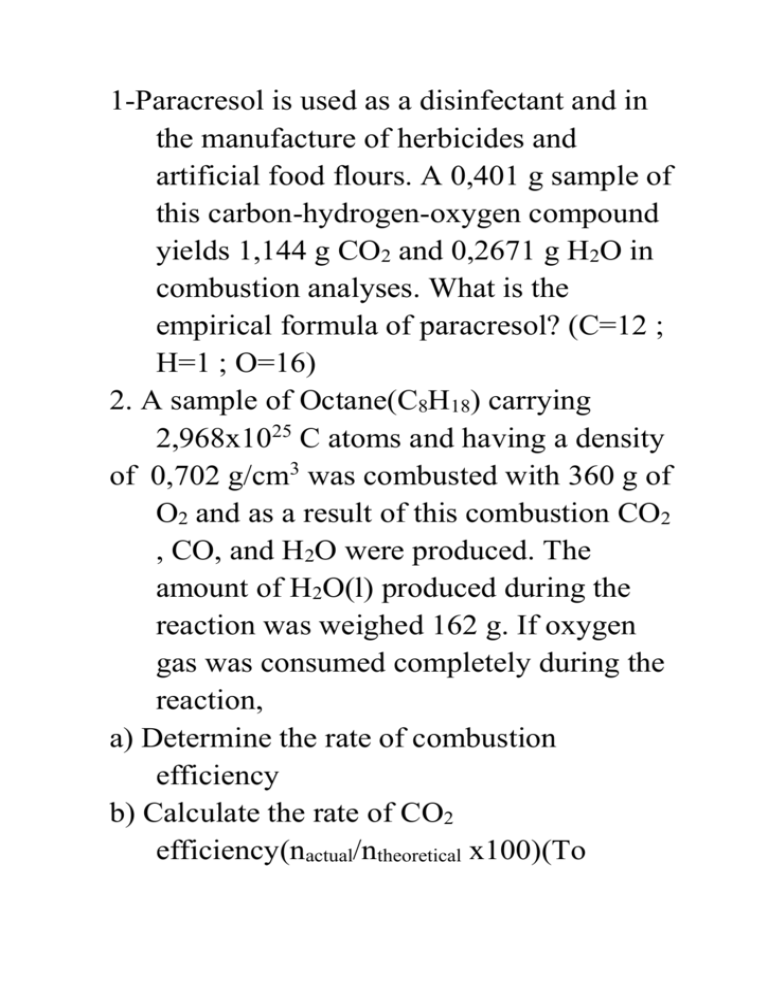
1-Paracresol is used as a disinfectant and in the manufacture of herbicides and artificial food flours. A 0,401 g sample of this carbon-hydrogen-oxygen compound yields 1,144 g CO2 and 0,2671 g H2O in combustion analyses. What is the empirical formula of paracresol? (C=12 ; H=1 ; O=16) 2. A sample of Octane(C8H18) carrying 2,968x1025 C atoms and having a density of 0,702 g/cm3 was combusted with 360 g of O2 and as a result of this combustion CO2 , CO, and H2O were produced. The amount of H2O(l) produced during the reaction was weighed 162 g. If oxygen gas was consumed completely during the reaction, a) Determine the rate of combustion efficiency b) Calculate the rate of CO2 efficiency(nactual/ntheoretical x100)(To calculate ntheoretical, assume that no CO was produced) c) Calculate the mass of O2 required to produce CO when the whole sample is burnt d) Calculate the volume of the sample in L (C=12 ; H=1 ; O=16) 2. Hexachlorophene, used in making germicidal soaps, has the following percent composition by mass: 38,77% C ; 1,49% H; 52,28% Cl ; 7,86% O. What is the empirical formula of hexachlorophene? 3- What mass of BHA(C11H16O2) should be burned to produce 0,5 g CO2 in combustion analysis? (C=12 ; H=1 ; O=16) 4- Name the compounds CaSO4 ∙ 2 H2O CaSO4 ∙ ½ H2O Mg SO4 ∙ 7 H2O Na2CO3 ∙ 10 H2O Na2B4O7 ∙10 H2O