ChemWork6
advertisement

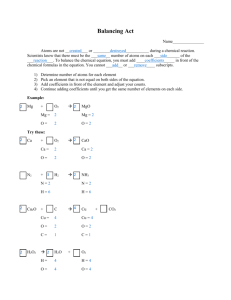
Chemistry Worksheet # 6 Do this worksheet after viewing the “Chem 6 Chemical Reactions” PowerPoint. Answer questions on your own paper. You do not need to write out questions, and you do not need to answer in sentences. 1. Balance these equations so that you begin and end the reaction with the same number of atoms. A. H2 + O2 >>>>>> H2O C. Mg + Cl2 >>>>>> MgCl2 B. H2 + C >>>>>> H2C6 D. Na + Cl2 >>>>>> NaCl 2. Which molecule below is diatomic? A. HCl C. H2O B. Cl2 D. NaCl 3. Which of the below are exothermic and which are endothermic reactions? A. Baking a cake C. Nuclear fission in a star B. Plants making food D. Lightning Bug glowing 4. Complete the chart below: Cellular Respiration Photosynthesis What happens in reaction? Who does it? Exothermic or Endothermic? Reactants (Chemicals Needed) Products (Chemicals Produced) 5. Write the equation for Cellular Respiration. 6. Write the equation for Photosynthesis. 7. What energy do plants need to make glucose, and how do they trap the energy? 8. Where is the energy stored in a molecule of glucose? 9. In photosynthesis, where does the carbon in the CO2 end up? 10. Why do plants produce oxygen?