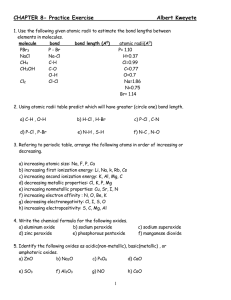
groups 1, 2 & 7 reactions.
advertisement
AS CHEMISTRY UNIT 2 - PIGUY GROUPS 1&2 >>REVISION>> FLAME TESTS Li RED Na ORANGE K LILAC Rb RED-VIOLET Cs BLUE electron is promoted to higher energy level by heat; when it drops down, energy is given off as light Mg Ca Sr Ba WHITE BRICK RED CRIMSON RED APPLE COX GREEN Mg(s) + O2 (g) 2MgO(s) Ba & Sr also form peroxides (e.g. BaO2) ɣ e- GROUP II REACTIONS CaCl2(s) + 2H2O (l) Mg doesn’t react with H2O (l) Be / BeO doesn’t react with either H2O (l) or (g) + Cl2 (aq) CaCl2(s) + H2O(l) + dil. 2HCl Ca(s) + 2H2O (l) + dil. 2HCl Ca(OH)2 (s) + H2 (g) + H2O (g) + H2O (l) CaO(s) + H2 (g) GROUP I & II TRENDS down the group the solubility of sulphates decrease hydroxides increase T H E R M A L S TA B I L I T Y C-O BOND EASIER TO BREAK O C O down the group the thermal stability of nitrates and carbonates increase Smaller positive ion means greater charge density Increase in ability to pull the O, weakening the C-O bond, so less thermal energy required O- M g 2+ OO C O B a 2+ AS CHEMISTRY UNIT 2 - PIGUY GROUP 7 >>REVISION>> PROPERTIES OF GROUP VII ELEMENTS GOOD OXIDISING AGENTS I2 > Br2 > Cl2 Cl2 in solution Br2 in solution I2 in solution PROPERTIES OF HALIDES GOOD REDUCING AGENTS C l -> B r - > I - S O D I S P L A C E M E N T O C C U R S 2KBr + Cl2 (aq) 2KCl + Br2 (aq) PA L E G R E E N G A S PA L E G R E E N S O L U T I O N DARK RED LIQUID ORANGE SOLUTION GREY SOLID with AgNO AgCl colour of ppt. white cream yellow dissolves in PURPLE SOLUTION * I O D I N E O N LY D I S S O LV E S I N N O N POLAR SOLUTIONS, NOT WATER 2AgCl (s) dil. NH AgBr conc. NH sunlight AgI - 2Ag + Cl2 GROUP VII ELEMENT REACTIONS halogens act as oxidising agents e.g. 2FeCl3 2Fe + 3Cl2 (aq) DOWN THE GROUP THEIR LESS GOOD AT OXIDISING SO IODINE GOES: Fe + I2 FeI2 DISPROPORTIONATION 2NaOH (aq) + Cl2 (aq) hot NaCl + NaClO + H2O 6NaOH(aq) + 3Cl2 (aq) cold 5NaCl + NaClO3 + 3H2O GROUP VII IONS REACTIONS halides act as reducing agents e.g. H 2S O 4 R E D O X ( D O E S N ’ T W O R K F O R C l -) 2BrH2SO4 + 2H+ + 2eH2SO4 + 8H+ + 8e- Br2 + 2eSO2 + 2H2O H2S + 4H2O EGG SMELL * O N LY F O R IODIDE HYDROGEN HALIDE REACTIONS HCl (g) + NH3 (aq) NH4Cl (aq) HCl (g)+ H2O (l) H3O+ + Cl- WHITE SMOKE YOU’RE DONE WITH INORGANIC CHEMISTRY REVISION!