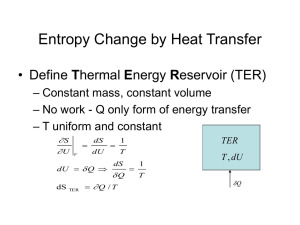
Entropy For a reversible ideal gas Carnot cycle: Efficiency The
advertisement

Entropy For a reversible ideal gas Carnot cycle: Efficiency = q rev T W 1 2rev 1 2 qrev q1 T1 q1 q2 0 T1 T2 dqrev 0 T The efficiency of any reversible engine has to be the same as the Carnot cycle: Since the engine is reversible, we can run it backwards. Use the work (-w’) out of the Cannot engine as work input (w) to run the left engine backwards. But ' Total work out = 0 (-w’ = w > 0) W ' W W W W ' q1 q1' since q1 0, q1' 0 ' q1 q1 q1 q1 q1 (q1' +q1 )>0 nd This contradicts the 2 law (Clausius). This says that we have a net flow of heat into the hot reservoir, but no work is being done! ∴ The efficiency of any reversible engine is 1 T2 T1 We can approach arbitrarily closely to any cyclic process using a series of only adiabats and isotherms. ∴ For any reversible cycle dqrev 0 T This defines Entropy, a function of state dS Note: 2 dq dqrev rev S S 2 S1 1 T T Entropy is a state function, but to calculate ΔS requires a reversible path. An irreversible Carnot (or any other) cycle is less efficient than a reversible one. ** An irreversible isothermal expansion requires less heat than a reversible one. irrev q2rev q2rev 1 irrev 1 irrev rev q1 q1 dqirrev dqrev also T T (q2 0) dqirrev 0 T ** Leads to Clausius inequality dq 0 T dqrev T 0 contains dqirrev 0 T The entropy of an isolated system never decreases (A): The system is isolated and irreversibly (spontaneously) changes from [1] to [2] (B): The system is brought into contact with a heat reservoir and reversibly brought back from [2] to [1] Path (A): qirrev = 0 Clausius (isolated) 2 dq dq irrev 0 1 T T 0! 1 2 dqrev 0 T dqrev S1 S 2 S 0 2 T ∴ 1 S S2 S1 0 This gives the direction of spontaneous changel S S2 S1 But! independent of path ΔSsurroundings depends on whether the process is reversible or irreversible (a) Irreversible: Consider the universe as an isolated system containing our initial system and its surroundings. (b) Reversible: Examples of a spontaneous process Connect two metal blocks thermally in an isolated system (ΔU = 0) Initially T1 T2 dS dS1 dS2 dq1 dq2 (T T ) dq1 2 1 T1 T2 T1T2 (dq1 dq2 ) dS 0 for spontaneous process if T2 T1 dq1 0 T2 T1 dq1 0 in both cases heat flows from hot to cold as expected Joule expansion with an ideal gas adiabatic mol gas (2V,T) 1 mol gas (V,T) ΔU = 0 q = 0 w = 0 S Sbackwards Compress back isothermally and reversibly qrev 0 1 mol gas (2V,T) = 1 mol gas (V,T) Sbackwards v RdV dqrev dw 1 R ln 2v V T T 2 ∴ S R ln2 0 spontaneous Note that to calculate ΔS for the irreversible process, we needed to find dqrev dq and rev a reversible path so we could determine T