Second Law of Thermodynamics
advertisement

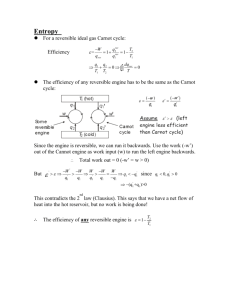
ERT 108 Physical Chemistry The Second Law of Thermodynamics by Miss Anis Atikah binti Ahmad anisatikah@unimap.edu.my Outline • • • • • • • The Second Law of Thermodynamics Heat Engines Entropy Calculation of entropy changes Entropy, Reversibility and Irreversibility The thermodynamics temperature scale What is entropy? The Second Law of Thermodynamics • Kelvin-Plack formulation of the second law of thermodynamics: It is impossible for a system to undergo cyclic process whose sole effects are the flow of heat into the system from a heat reservoir and the performance of an equivalent amount of work by the system on the surroundings. The Second Law of Thermodynamics Does this system violate the first law? It is impossible to build a cyclic machine that converts 100% heat into work. The Second Law of Thermodynamics Any heat engine must eject heat into the cold reservoir Heat Engines • Heat engine: a device that operates in a thermodynamic cycle and does a certain amount of net positive work as a result of heat transfer from a high-temperature body to a lowtemperature body. (eg: the internal-combustion engine and the gas turbine) Hot reservoir Heat Engines Cold reservoir • The efficiency of heat engine: Work output per cycle Energy input per cycle wcycle qH qH qC qC 1 qH qH • The efficiency value is less than 1, qC has a negative value and qH has a positive value. Heat Engines The cycle for a reversible heat engine (Carnot cycle): Heat, Work and ΔU for Reversible Carnot Cycle Work flow in Carnot cycle Carnot cycle • For a complete cycle (assuming perfect gas); dU dq dw First Law dq PdV CV dT dq nRT V dV • Dividing by T and integrating over Carnot cycle; dT dq dV CV T T nR V Carnot cycle Differential of state function; independent of the path taken to reach final state. 0 0 dT dq dV CV T T nR V • Thus; dq T 0 b c d a dq dq dq dq dq T a T b T c T d T 0 Carnot cycle • Since bc and da are adiabatic; dq=0; 0 b c b d d a 0 dq dq dq dq dq T a T b T c T d T 0 • Thus; dq dq dq T a T c T 0 dq qH qC T TH TC 0 Carnot cycle For Carnot cycle the efficiency can be also written as; rev Work output per cycle Energy input per cycle qC TC 1 1 qH TH Because qC / qH TC TH Where rev is the maximum possible efficiency for the conversion of heat to work. Exercise 1 • Calculate the maximum work that can be done by reversible heat engine operating between 500 and 200 K if 1000 J is absorbed at 500 K Solution • Calculate the maximum work that can be done by reversible heat engine operating between 500 and 200 K if 1000 J is absorbed at 500 K qC TC 200 K 1 1 1 0.6 qH TH 500 K w qH 0.61000J 600J Entropy, S dqrev dS T 2 dqrev S S 2 S1 T 1 Closed sys, rev. process Calculation of Entropy Changes 1. Cyclic process; S 0 2. Reversible adiabatic process; dqrev0 S 0 T 1 S 0 2 Rev. adiab. proc. 3. Reversible phase change at constant T & P 2 2 dqrev 1 qrev S dqrev T T 1 T 1 at constant P, qrev qP H , thus S T Rev. phase change at const. T & P Calculation of Entropy Changes 4. Reversible isothermal process 2 S qrev T 2 dqrev 1 qrev S dqrev T T 1 T 1 Rev, isothermal proc. 5. Constant-pressure heating with no phase change 2 2 dqrev dqP S T T 1 1 T2 CP S dT T T1 If Const. P, no phase change qP CP dT C P is constant over the temperature range, then S CP ln T2 T1 Calculation of Entropy Changes 6. Reversible change of state of a perfect gas; dqrev dU dw CV dT PdV CV dT nRT V dV 2 S 1 2 dqrev T 2 CV dT nR V dV T 1 1 2 CV V dT nR ln 2 T V1 1 Perfect gas Calculation of Entropy Changes 7. Irreversible change of state of a perfect gas; 2 CV V2 S dT nR ln T V1 1 Perfect gas 8. Mixing of different inert perfect gases at constant T & P S S1 S2 na R ln V Va nb R ln V Vb na R ln xa nb R ln xb V na nb RT P Va na RT P V Va na nb RT 1 xa na RT P P Exercise 2 One mole of a perfect gas at 300 K is reversibly and isothermally compressed from a volume of 25.0 L to a volume of 10.0 L. Because the water bath in the surroundings is very large, T remains essentially constant at 300 K during the process. Calculate ΔS of the system. Exercise 2-Solution One mole of a perfect gas at 300 K is reversibly and isothermally compressed from a volume of 25.0 L to a volume of 10.0 L. Because the water bath in the surroundings is very large, T remains essentially constant at 300 K during the process. Calculate ΔS. • This is an isothermal process, ΔT=0, thus ΔU=0 (for perfect gas, U depends only on T. ( dU CV dT ) U q w 0 qrev w PdV nRT dV nRT ln V2 V1 V 1mol 8.314 J mol 1 K 1 300K ln 10L 25 L 2.285 103 J qrev 2.285 103 J S 7.62 J K 1 T 300 K Exercise 3 Calculate ΔS for the melting of 5.0 g of ice (heat of fusion= 79.7 cal/g) at 0°C and 1 atm. Estimate ΔS for the reverse process Exercise 3- Solution Calculate ΔS for the melting of 5.0 g of ice (heat of fusion= 79.7 cal/g) at 0°C and 1 atm. Estimate ΔS for the reverse process. • Identify type of process: ▫ Phase change at constant T & P ▫ At constant P, q= ΔH ▫ Thus, S T • Calculate ΔS; 79.7 cal g 5 g S 1.46 cal K 6.1 J K T 273.15K Exercise 3- Solution Calculate ΔS for the melting of 5.0 g of ice (heat of fusion= 79.7 cal/g) at 0°C and 1 atm. Estimate ΔS for the reverse process. • ΔS for reverse process (freezing of 5g liquid water ); S 6.1 J K Exercise 4 The specific heat capacity cP of water is nearly constant at 100 cal/g K in the temperature range of 25°C to 50°C at 1 atm. (a) Calculate ΔS when 100 g of water is reversibly heated from 25°C to 50°C at 1 atm. (b) Without doing a calculation, state whether ΔS for heating 100g of water from 50°C to 75°C at 1 atm will be greater, equal to or less than ΔS for the 25°C to 50°C heating. Exercise 4- Solution The specific heat capacity cP of water is nearly constant at 100 cal/g K in the temperature range of 25°C to 50°C at 1 atm. (a) Calculate ΔS when 100 g of water is reversibly heated from 25°C to 50°C at 1 atm. 2 2 dqrev dqP S T T 1 1 CP mcP 100g 1.00 cal g K 100 cal K T2 CP 323K dT CP ln 100 cal K ln T 298K T1 T1 T2 33.7 J K Exercise 4 - Solution The specific heat capacity cP of water is nearly constant at 100 cal/g K in the temperature range of 25°C to 50°C at 1 atm. (b) Without doing a calculation, state whether ΔS for heating 100g of water from 50°C to 75°C at 1 atm will be greater, equal to or less than ΔS for the 25°C to 50°C heating. 2 dqrev S T 1 S 1 T Thus, ↑T, ↓ΔS ΔS for heating 100g of water from 50°C to 75°C at 1 atm will be smaller than ΔS for the 25°C to 50°C heating. Entropy, Reversibility and Irreversibility • Reversible Process, ΔSuniv = 0 dSuniv dS system dS surr dqrev dqrev Tsys Tsurr dqrev dqrev Tsys Tsys 0 In reversible process, any heat flow btween system & surroundings must occur with no finite temperature difference Entropy, Reversibility and Irreversibility • Irreversible Process, ΔSuniv > 0 Recall first law; dU dq dw dqrev dwrev rearranging dqrev dq dw dwrev More work is done when a change is reversible than when it is irreversible; dwrev dw When energy leaves the system as work, dwrev dw rearranging dw dwrev 0 Entropy, Reversibility and Irreversibility • Irreversible Process, ΔSuniv > 0 Substituting dw dwrev 0 into dqrev dq dw dwrev dqrev dq 0 dqrev dq Dividing by T; dqrev dq T T dq dS T Clausius inequality Entropy, Reversibility and Irreversibility • Irreversible Process, ΔSuniv > 0 Suppose that the system is isolated from its surroundings, thus dq=0 dq dS T S sys surr Suniv 0 dS 0 Entropy, Reversibility and Irreversibility • Entropy & Equilibrium S Equilibrium reach S=Smax Time Thermodynamic equilibrium in an isolated system is reached when the system’s entropy is maximized. The thermodynamics temperature scale -a scale that is independent of the choice of a particular thermometric substance. T 1 C TH rearranging TC 1 TH This expression enabled Kelvin to define thermodynamic temperature scale Kelvin scale is defined by using water at its triple point as the notional of hot source and defining that temperature as 273.16 K If it is found that the efficiency of heat engine equal to 0.2, then the temperature of cold sink is (0.8) x 273.16 K =220 K, regardless of the working substance of the engine. What is entropy? • Entropy is a measure of the probability, p of the thermodynamic state a a a a b b b b a a a a b b b a b a b b b a b a Partition removed System proceed to equilibrium Irreversible mixing of perfect gas at constant T & P The probability that all a molecules will be in the left half & all b molecules in right half is extremely small. The most probable distribution has a and b molecules equally distributed. What is entropy? • Entropy is a measure of molecular disorder of a state. Eg: In mixing two gases, the disordered (mixed state) is far more probable than the ordered (unmixed) state. What is entropy? • Entropy is related to the distribution or spread of energy among the available molecular energy levels. • The greater the number of energy levels, the larger the entropy is. • Increasing the system’s energy (eg:by heating) will increase its entropy because it allows higher energy levels to be significantly occupied • Increasing the volume of a system at constant energy also allows more energy level to be occupied. What is entropy? • Boltzmann made link btween distribution of molecules over energy levels and the entropy; S k ln W Where k= 1.381 x 10-23 JK-1 W= probability/ways in which the molecules of a system can be arranged while keeping the energy constant Exercise • True or false? ▫ ΔSuni for a reversible process in a closed system must be zero ▫ ΔS for a reversible process in a closed system must be zero ▫ For a closed system, equilibrium has been reached when S has been maximized. • What is ΔSuni for each steps of a Carnot cycle?