PPT - Huntingdon College
advertisement

Entropy, the Second and Third
Law of Thermodynamics
By
Doba Jackson, Ph.D.
Associate Professor of Chemistry and Biochemistry
Huntingdon College
Outline of Chpt 3
• Sec 3.1-2: The Universe has a natural
direction of change
• Sec 3.3: Heat Engines & Second Law
• Sec 3.4: Introducing Entropy
• Sec 3.5: Entropy to calculate natural
direction of change
Outline of Chpt 3
• Sec 3.6: Absolute Entropies and the Third
Law of Thermodynamics
• Sec 3.7: Standard Molar Entropies
• Sec 3.7 con’t: Entropy changes in
chemical reactions
The Universe has a Natural
Direction of change
Why ?
Reminder of microscopic
definition of Heat and Work
Energy released as Heat
Energy produces useful work
Direction of Spontaneous
change
Bouncing ball demonstrates the
direction of spontaneous change.
System: The Ball
Surroundings: The Floor
- The internal energy of the ball depletes
after each collision with the floor.
- Some energy is converted to work on the
ball which causes the ball to rise.
- Other energy is dispersed into the floor
(as heat).
Direction of Spontaneous
change
The bouncing ball spontaneously bounces
toward a state of lower energy until all of
its energy has been dissipated into the
floor.
System: The Ball
Surroundings: The Floor
- By the first law, total energy for the ball
colliding with the floor is conserved.
-However, ordered motion (work) is
gradually converted to disordered motion
(heat)
Sec 5.2: Heat Engines and the
Second law of Thermodynamics
Argument for new
Thermodynamic law
• Saudi Carnot (in 1824) argued for a new law based
upon many studies on how to improve a heat engine.
Argument for new
Thermodynamic law
• Saudi Carnot (in 1824) argued for a new law based
upon many studies on how to improve a heat engine.
Carnot Cycle
Cyclic Process:
A-B: Isothermal Expansion
B-C: Adiabatic Expansion
C-D: Isothermal Compression
D-A: Adiabatic Compression
Carnot Cycle
A-B: Isothermal Expansion
U 0 W = -nRT Ln VB q = nRT Ln VB
AB
AB
AB
AB
VA
VA
q = -W
B-C: Adiabatic Expansion
q=0
U = W
U BC = CV ΔT
WBC = CV ΔT
C-D: Isothermal Compression
U 0
q = -W
WCD
VD
VD
= -nRTCD Ln
q CD = nRTCD Ln
VC
VC
D-A: Adiabatic Compression
q=0
U = W
U DA = CV ΔT
WDA = CV ΔT
Net balance of energy
• Net ∆U:
UT U BC U DA
ΔUT = CV TCD - TAB + CV TAB - TCD
UT 0 State Function
• Net Work (W):
WT = WAB + WBC + WCD + WDA
VB
VD
WT = -RTAB Ln
+ CV TAB - TCD + -RTCD Ln
VA
VC
VB
VD
WT = -RTAB Ln
+ -RTCD Ln
VA
VC
VB
VA
WT = -RTAB Ln
+ -RTCD Ln
VA
VB
+ CV TCD - TAB
VB VC
VA VD
VB
WT TCD - TAB RLn
VA
• Net Heat (q):
qT = q AB + qCD
VB VC
VB
VD
qT = RTAB Ln
+ RTCD Ln
VA VD
VA
VC
VB
VB
VB
qT = RTAB Ln
+ -RTCD Ln
q T = TAB - TCD RLn
VA
VA
VA
TAB = Th
Heat
Work
Work
Heat
TCD = Tc
Carnot and others were trying to
make the engine more efficient
Heat engine Efficiency
WT
Work done
ε=
=
Heat input
qh
ε=
-R(Th - Tc )Ln
R(Th )Ln
Vf
Vf
Vi
Vi
Th Tc
ε=
Th
Only way to reach 100%
efficiency is to make Tc = 0.
Carnot tried to improve the
heat engine by coupling a
second engine capable of
working in reverse
This was a failure!!
Carnot’s Theorems
1- All reversible heat engines operating
between two reservoirs are equally
efficient as a Carnot engine operating
between the same reservoirs.
2- All irreversible heat engines operating
between two reservoirs are equally
efficient as a Carnot engine between
the same reservoir.
Problem 1: A turbine in a steam power
plant takes in steam from a boiler at
520*C and exhaust it into a condenser
at 100*C. What is the maximum
possible efficiency?
Th Tc 793 K 373 K
.529
Th
793 K
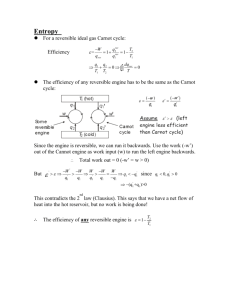
Sec 5.3: Introducing Entropy
Entropy
WT
Work done
ε=
=
Heat input
qh
WT = WAB + WBC + WCD + WDA
WT = |WAB + WCD |= |q h + qc |
qh + qc
ε=
qh
Th Tc
ε=
Th
qc
qh
Tc
Th
Later Lord Kelvin postulated that the ratio of heat
to temperature was a state function and called it
entropy.
Second Law of Thermodynamics
Typical Engine
Hypothetical Engine
Spontaneous nature of heat
Spontaneous
Not Spontaneous
Refrigeration
Introduction to Entropy
Spontaneous Process: A process that, once started,
proceeds on its own without a continuous external
influence.
Entropy (S): The amount of molecular randomness in
a system.
Spontaneous processes are
•
•
favored by a decrease in H (negative ∆H).
favored by an increase in S (positive ∆S).
Nonspontaneous processes are
•
•
favored by an increase in H (positive ∆H).
favored by a decrease in S (negative ∆S).
Enthalpy term is
larger than Entropy
Entropy term is
larger than Enthalpy
Sec 5.4, 5.5: Calculating
Entropy changes
Calculating Entropy changes
• Mathematical Statement of the Second Law
dqrev
0
T
The sum of the ratio of heat to
temperature for any reversible
cyclic process will be zero.
• Adiabatic, Reversible process
qrev 0
S
i
f
dqrev
0
T
Calculating Entropy changes
of an Ideal Gas
• Isothermal Expansion, reversible
U 0
q = nRTLn
S
i
f
Vf
q = -W
Vi
dqrev
1
qrev
T
T
nRT ln
Vf
ΔS = nR Ln
Vi
T
W = -nRT Ln
Vf
Vi
Vf
Vi
Calculating Entropy changes
of an Ideal Gas
• Constant Volume, reversible
wrev 0
U qrev nCV T
S
i
f
f nCV , m dT
Tf
dqrev
nCV ,m ln
i
T
T
Ti
S nCV ,m ln
Tf
Ti
Calculating Entropy changes
of an Ideal Gas
• Constant Pressure, reversible
U qrev nCP T
S
i
f
wrev 0
f nC P , m dT
Tf
dqrev
nCP ,m ln
i
T
T
Ti
S nC P ,m ln
Tf
Ti
Calculating Entropy changes
when a phase change occurs
• Phase changes occur under constant
temperature and pressure conditions
Calculate the change in entropies of the system,
surroundings and the universe when a sample of
nitrogen gas of mass 14 g at 298 K and 1.00 bar
doubles its volume in
(a) an isothermal reversible expansion
(b) an isothermal irreversible expansion against a constant
external pressure of P=0
(c) an adiabatic reversible expansion.
Sec 5.6: The Clausius
Inequality
Types of Expansion Work
• Expansion against a constant pressure,
irreversible
Vf
w Pex dV
Vi
If external pressure does, not vary,
this expression can be integrated
Vf
w = -Pex dV
V1
w = -Pex Vf -Vi
w = -Pex ΔV
Types of Expansion
Work
• Expansion is reversible
Vf
w Pex dV
Vi
In this case, the external pressure Pex
exactly equals the internal pressure P
at each stage of the expansion.
Vf
w = - P dV
Pex = P
V1
w = -
Vf
Vi
nRT
dV
V
The integral cannot be evaluated because
temperature is not a constant throughout the
integration.
Clausius Inequality
Reversible
dU dq dw
Irreversible
dwrev dw
dU dq dw dqrev dwrev
dq dqrev dwrev dw
dwrev dw 0
dqrev dq
T
T
dq
S
T
An irreversible process produces
less work and heat than a reversible
process
dq dqrev 0
Reversible, Nonspontaneous
Reversible Process: The thermodynamic process in which a system
can be changed from its initial state to its final state then back to its initial
state leaving all thermodynamic variables for the universe (system +
surroundings) unchanged.
A truly reversible change will:
- Occur in an infinite amount of time
- All variables must be in equilibrium with each
other at every stage of the change
Reversible
Irreversible
Irreversible, Spontaneous
Irreversible Process: The thermodynamic process in which a system
that is changed from its initial state to its final state then back to its initial
state will change some thermodynamic variables of the universe.
A truly irreversible change will:
- Occur in an finite amount of time
- All variables will not be in equilibrium with each
other at every stage of the change
Reversible
Irreversible
Clausius Inequality leads to
equations for spontaneity
dwrev dw 0
dS
dq
T
dqrev
dq
T
T
TdS dq
Constant Volume
TdS dqV
TdS dU
dA dU TdS
Helmholtz Energy
A U T S
dA > 0; Reversible, not spontaneous
dA = 0; Equilibrium
dA < 0; Irreversible, Spontaneous
Reversible
Irreversible
dq dqrev 0
Clausius Inequality leads to
equations for spontaneity
TdS dq
Reversible
Constant Pressure
TdS dq p
TdS dH
dG dH TdS
Gibbs Energy
G H T S
dG > 0; Reversible, not spontaneous
dG = 0; Equilibrium
dG < 0; Irreversible, Spontaneous
Irreversible
Sec 5.7: The Change in
Entropy of the surroundings
and Universe
Problem 5.7: One mole of an Ideal gas at 300 K is
reversibly and isothermally compressed from a volume of
25.0 L to a volume of 10.0 L. Because the water bath’s
thermal reservoir in the surroundings is very large, the
temperature remains relatively constant at 300 K. Calculate
∆S, ∆Ssurr ∆Stot .
S sys
Vf
10.0
nR ln
1 mol 8.314 J / K mol ln
.762 J / K
Vi
25.0
S sys .762 J / K
S surr .762 J / K
Stot 0 ; reversible process
Problem 5.7: One mole of an Ideal gas at 300 K is
isothermally compressed at a constant external pressure
from a volume of 25.0 L to a volume of 10.0 L. At the end of
the compression, the internal and external pressures are
equal. Calculate ∆S, ∆Ssurr ∆Stot .
S sys
Vf
10.0
nR ln
1 mol 8.314 J / K mol ln
.762 J / K
Vi
25.0
S sys .762 J / K
Since Entropy is a state function, the initial and final states of the system
are the same, so the entropy change of the system is the same. However, the
surroundings and total entropy will change because the process is irreversible.
Problem 5.7: One mole of an Ideal gas at 300 K is isothermally
compressed at a constant external pressure from a volume of
25.0 L to a volume of 10.0 L. At the end of the compression,
the internal and external pressures are equal. Calculate ∆S,
∆Ssurr ∆Stot .
Since ∆U = 0, isothermal process
nRT
Pex
Vf
3
Pa
m
1 mol 8.314
mol K
.0100 m 3
300 K 2.494 x 10 Pa
5
qirr wirr Pex V 2.494 x105 Pa .0100 m3 .0250 m3
qirr wirr Pex V 3.741 x103 J
Work and heat are not state functions so they will be different for the irreversible
process. We can calculate the entropy of the surroundings by first calculating the
Work done by the surroundings.
qsurr 3.741 x103 J
Problem 5.7: One mole of an Ideal gas at 300 K is
isothermally compressed at a constant external pressure
from a volume of 25.0 L to a volume of 10.0 L. At the end of
the compression, the internal and external pressures are
equal. Calculate ∆S, ∆Ssurr ∆Stot .
S sys .762 J / K
S surr
qsurr 3.741 x103 J
12.5 J / K
T
300 K
Stot S sys S surr .762 J K 12.5 J K 11.7 J K
The total entropy change of the universe is positive even when the entropy
of the system is negative.
Problem 5.9:
Calculate ΔS, ΔStot, Δssur when a volume of 150 g
of CO initially at 273 K and 1.00 bar increases by a
factor of two in:
(a) Adiabatic reversible expansion
(b) expansion against a Pext =0
(c) isothermal reversible expansion
Take Cp,m as a constant at 29.14 J/K*mol and state
whether each process is spontaneous. The
temperature of the surroundings is 273 K for each
process.
Entropy change during
Temperature change
Heat required to
change the temperature
S T1 S T2
T2
T1
At Constant Pressure
T2
C p dT
T1
T
S T1 S T2
S T1 S T2 C p Ln
dqrev
T
Tf
Ti
At Constant Volume
S T1 S T2 CV Ln
Tf
Ti
Assume a Constant Heat Capacity
Over the temperature range
Sec 5.7: Absolute Entropies
and the Third Law of
Thermodynamics
Entropy in Chemical
Thermodynamics
We rely on calorimetry to measure the internal energy (∆U)
enthalpy (∆H). We can also measure Entropy (∆S) for all
Chemical and Physical Changes.
Third Law of Thermodynamics
• At T=0, all thermal motion has been quenched and in
a perfect crystal, all atoms are in a uniform array.
• Third Law: The entropy of a perfect crystalline
substance is zero at T=0
Standard Entropies (Third Law
Entropies)
Perfect crystalline
Sm (0) = 0
Sm T = Sm 0 +
Tf
0
Cp,m s, T
T
dT
fus H
Tf
Tb
Cp,m l , T
Tf
T
dT
vap H
Tb
T
Cp,m g , T
Tb
T
Standard Molar Entropies are used to
determine reaction enthalpies (ΔrSº)
Reaction entropies (ΔrSº) can be determined by the
difference of the product entropies and the reactant
entropies.
Δr S 0 =
Products
Example:
v Sm0 -
H2(g) + ½ O2(g)
v S m0
Reactants
H2O(l)
∆rS° = { (1)Sm (H2O)} – {(1)Sm(H2) + (1/2)Sm(O2)}
Products
Reactants
= -163.4 kJ/mol