Notes-16
advertisement
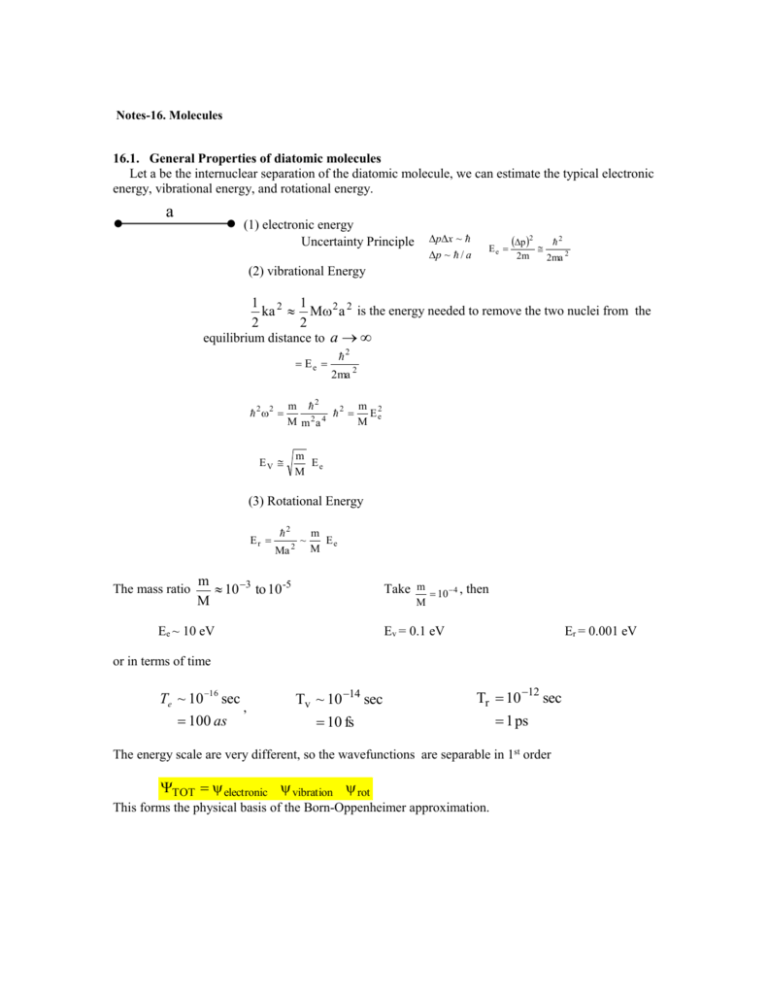
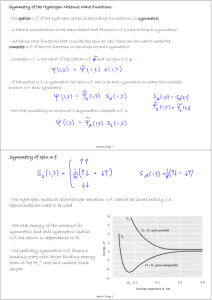
Notes-16. Molecules 16.1. General Properties of diatomic molecules Let a be the internuclear separation of the diatomic molecule, we can estimate the typical electronic energy, vibrational energy, and rotational energy. a (1) electronic energy Uncertainty Principle p x ~ p ~ / a Ee p 2 2m 2 2ma 2 (2) vibrational Energy 1 2 1 ka M 2 a 2 is the energy needed to remove the two nuclei from the 2 2 equilibrium distance to a Ee 2 2 2 2ma 2 m 2 m 2 E e2 M m 2a 4 M EV m Ee M (3) Rotational Energy Er The mass ratio 2 Ma 2 ~ m Ee M m 10 3 to 10 -5 M Take m 10 4 , then M Ee ~ 10 eV Ev = 0.1 eV Er = 0.001 eV or in terms of time Te ~ 10 16 sec 100 as , Tv ~ 10 14 sec Tr 10 12 sec 10 fs 1 ps The energy scale are very different, so the wavefunctions are separable in 1st order TOT electronic vibration rot This forms the physical basis of the Born-Oppenheimer approximation. 16.2. The Born Oppenheimer (BO) approximation for one-electron diatomic molecules. e- r R MA MB MAMB MA MB In the center-of-mass frame of MA and MB, the Schrödinger is TN Te V R, r E R, r TN 2 2 R 2 Te 2 2 r 2m (1) V : total Coulomb Interactio n Treat the electronic motion at fixed R Te V q R; r E q R qR; r (2) q : electronic wave function, R is fixed in this equation. Born-Oppenheimer expansion R, r Fq R q R; r (3) q Substitute (3) into (1) and use (2), we obtain dr *s TN Te V E Fq R q 0 (4) q Note TN Fq q 2 Fq 2R q 2 R Fq R q q 2R Fq 2 (5) From which 2 2 R E s R E Fs R s 2R q Fq 2 s R q R Fq 0 2 q (6) If the coupling terms are neglected, assuming that the change of the electronic motion with respect to R is small, then 2 2 R E s R E Fs R 0 2 is to be solved for the nuclear motion. Note (7) 2 2 2 1 2 N2 TN R R 2 2 R 2 2R R 2R 2 where N2 is the square angular momentum operator for the internuclear rotational motion. (8) Let 1 Fs R FsJ R YJMJ , R (9) where , are spherical angles of R with respect to the Lab-fixed axis, then 2 d 2 2 J J 1 E s (R ) E s,,J Fs,J R 0 2 2 2R 2 dR (10) The total energy E s , ,J is specified by the electronic state s, vibrational state and rotational state J. Within the BO approximation, one has to solve (2) and (10). A typical potential Es(R) for a given J. The equilibrium distance Ro and dissociation energy De are defined as above. Typical Examples: H2 I2 H 2 O2 N2 Ro(oA) 0.74oA 2.66oA 1.06 De(eV) 4.75eV 1.56eV 2.65eV 1.21 1.09 5.08eV 9.75eV 16.3. The H 2 Molecule-The electronic wavefunction can be solved exactly within the BO approximation. 3.1 The “exact” solution rA The electronic Hamiltonian A H+ 1 1 1 1 H e 2r 2 r rB R A rA r R / 2 rB r R / 2 r rB B R H+ H e s R; r E s R s R; r This Hamiltonian is separable in elliptical spheroidal coordinates, but will not be treated here. 3.2. LCAO—Linear Combination of Atomic Orbitals We set to obtain approximate solutions starting with atomic orbitals. Note at large R we have R; r 1s rA or 1s rB depending on electron is close to A or B. Since the two states are degenerate, the zeroth order solution should be 1 1s rA 1s rB g R; r 2 1 1s rA 1s rB u R; r 2 (18) Using this model, the electronic potential is calculated from E g ,u g ,u H e g ,u g ,u | g ,u This gives a 1st order estimate of the potential energy at large R. Note g : A ψa : BR bonding A B anti-bonding 3.3. All that you need to know about molecular orbitals of diatomic molecules The molecular orbitals are central to the understanding of the electronic structure of molecules. They are the equivalent of atomic orbitals for atoms. We will focus only on diatomics here. The orbital energies, as well as the orbital wavefunctions, depend on the internuclear separation R. The molecular orbitals can be "constructed" from the linear combination of atomic orbitals. This set of drawings shows how the MO's of a homonuclear molecule are constructed from the + and - combination of two AO's from the two centers. The dashed lines are the nodal surfaces. F1. g&u states from two 2s AO's. F2. from two 2pz orbitals. F3. from two 2px orbitals F4. An alternative way of bring two AO's to form molecular orbitals.