Notes 12
advertisement
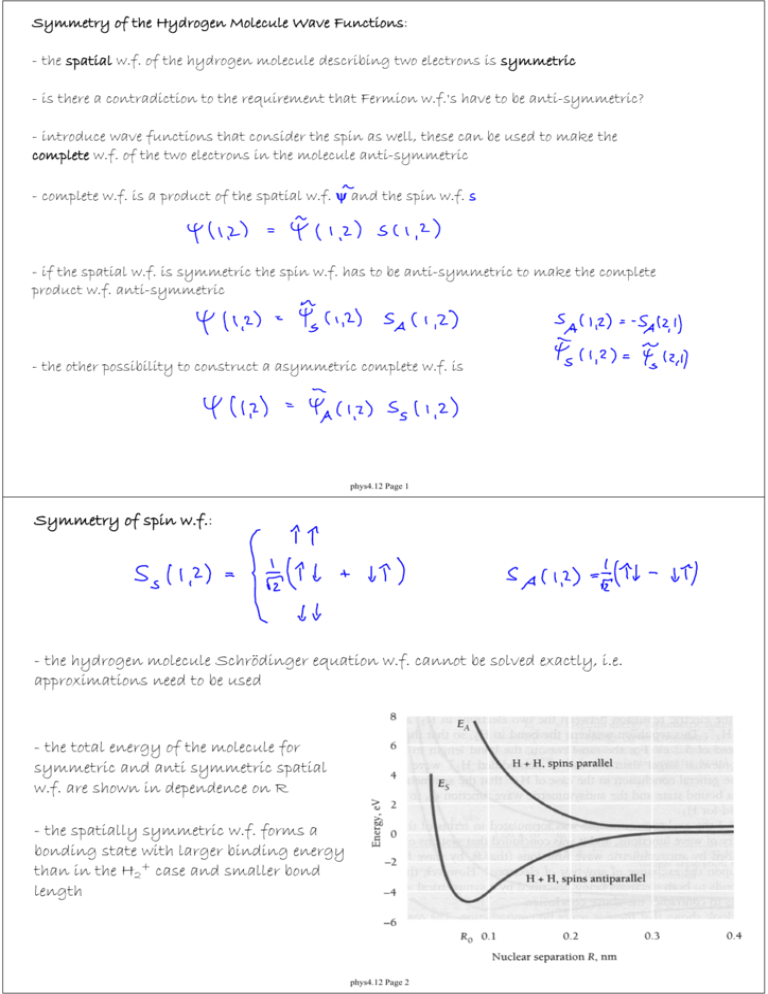
Symmetry of the Hydrogen Molecule Wave Functions: - the spatial w.f. of the hydrogen molecule describing two electrons is symmetric - is there a contradiction to the requirement that Fermion w.f.'s have to be anti-symmetric? - introduce wave functions that consider the spin as well, these can be used to make the complete w.f. of the two electrons in the molecule anti-symmetric - complete w.f. is a product of the spatial w.f. ψ and the spin w.f. s - if the spatial w.f. is symmetric the spin w.f. has to be anti-symmetric to make the complete product w.f. anti-symmetric - the other possibility to construct a asymmetric complete w.f. is phys4.12 Page 1 Symmetry of spin w.f.: - the hydrogen molecule Schrödinger equation w.f. cannot be solved exactly, i.e. approximations need to be used - the total energy of the molecule for symmetric and anti symmetric spatial w.f. are shown in dependence on R - the spatially symmetric w.f. forms a bonding state with larger binding energy than in the H2+ case and smaller bond length phys4.12 Page 2 Complex Molecules: - it is generally difficult to solve the quantum mechanical problem of diatomic or polyatomic molecules with many electrons - frequently it is sufficient to consider the effect of the interaction between atoms on the outermost electron shell - electrons in the outermost shell are called valence electrons - inner electrons do not contribute strongly to atom/atom interactions as they are more tightly bound by the nucleus, simultaneously repulsive atom/atom forces become important for separations R where inner shells are still relatively distant from each other - X-ray spectra confirm that inner shell electronic states are essentially independent of molecular bonding of the atoms - the exclusion principle determines which covalently bound molecules can exist - He2, H3 etc. do not exist - H2O does exist as a stable molecule phys4.12 Page 3 Molecular Bonds: - boundary surface diagrams - figure shows s, p = (px, py, pz) orbitals - bonds are formed when orbitals of bonding atoms overlap (obeying the exclusion principle ) - this overlap increases the probability density of electrons in between atoms resulting in an attractive force phys4.12 Page 4 Labeling of Molecular Orbitals: - greek letters indicate the total angular momentum along the bonding axis (usually the z axis) - in the hydrogen molecule H2 ssσ bonding orbitals are formed - pz orbitals form σ bonds - px, py orbitals form π bonds - atomic orbitals forming the bonds may be in different angular momentum states, as in H2O with two semi occupied 2p orbitals and two Hydrogen 1s1 orbitals phys4.12 Page 5 Hybrid Orbitals: - consider molecular structure of CH4 - is expected to form CH2 molecules with two spσ orbitals at 90 degrees - linear combination of two 2s2 and and two 2p2 orbitals into four sp3 orbitals - results in perfectly symmetric CH4 molecule with four equal C-H bonds - this effect is called hybridization, discovered by Linus Pauling in 1928 (Chemistry Nobel Prize 1954) - in presence of the outer H atoms the potential experienced by the electrons bound to the C nucleus is symmetrized to form new sp3 orbitals with equivalent energies - hybrid orbitals occur when the resulting bonding energies are lower then with unhybridized orbitals phys4.12 Page 6 - sp3 hybridized wave functions: - sp2 hybridization: one electron stays in a pure p state, the remaining three form hybridized orbitals (e.g. Ethylene, C2H4) - the bonds to the Hydrogen atoms are σ type - between the Carbon atoms there is one σ (see panel b) and one π bond (see panel c) phys4.12 Page 7 - another prominent sp2 hybridized molecule is benzene - the three sp2 orbitals per carbon atom form the bonds to the H and two neighboring C - the p orbital electrons form a delocalized π orbital in the hexagonal plane of the molecule - sp hybridization: two electrons stay in a pure p state, the remaining two form hybridized orbitals phys4.12 Page 8 Rotational Energy Levels in Molecules molecular energies depend on - electronic configuration - vibrations of bound atoms relative to each other - rotations of the molecule as a whole - electronic transitions: change of electronic state (as in atoms) on the energy scale of eV (visible, UV light transitions) - vibrational transitions: change of the vibration state on energy scale of 0.1 eV (infrared light) - rotational transitions: change of the rotational state on energy scale of 1 meV (microwave frequencies) - the detailed structure (bond lengths, bond angles etc.) of molecules can be extracted from their excitation spectra - here we only consider diatomic molecules for simplicity phys4.12 Page 9 Rotation of diatomic molecules: - consider rotation about center of mass coordinate - moment of inertia I - center of mass defined by - thus - with reduced mass m' and inter atomic distance R - the rotation of a diatomic molecule is equivalent to the rotation of a particle with mass m' about an axis at a distance R - rotational angular momentum - rotational angular momentum is quantized with rotational quantum number J phys4.12 Page 10 Rotation Energy: - rotational energy levels are determined by the rotational quantum number J exercise: Determine the rotational energy levels for rotations about the bonding axis of the molecule. What can one conclude from the result? Rotational Spectra: - only molecules with dipole moments exhibit electromagnetic rotational spectra (otherwise the rotational state can only be changed in collisions) - symmetric molecules such as CH4, CO2 do not have dipole moments - spectral lines are determined by transitions between different rotational states phys4.12 Page 11 - selection rules: - transition frequency: - the transition frequency ν is proportional to the rotational quantum number J - thus an equidistant set of rotational spectral lines is observed - from the rotational transition frequencies the moment of inertia I of the molecule can be determined, - knowing the masses of the constituents of the diatomic molecule the bond length R can be extracted phys4.12 Page 12 Vibrational energy levels of molecules: - molecules can be excited to vibrated along their bonds - the potential energy of the molecule varies with the elongation of the bond, see figure - the minimum in the potential energy corresponds to the ground state elongation of the bond (no vibrational excitation) - parabolic approximation of the vibration potential energy of molecule - k spring constant, R0 equilibrium separation of the atoms - Hooke's law: - i.e. the molecule vibrations behave like a harmonic oscillator, in first approximation the elongation of the bond of the molecule results in a linear restoring force phys4.12 Page 13 Frequency of Molecular Vibrations: - classical expression for body with mass m on a spring with spring constant k - situation is different for a diatomic molecule, two bodies (possibly of different mass) connected by a bond (spring) effective oscillator mass m' - quantum harmonic oscillator energies - vibrational quantum number - for molecular vibrations phys4.12 Page 14 Note: - the molecular oscillator has zero-point energy - in accordance with uncertainty principle - vibrational levels and rotational sublevels (for each vibrational state) are indicated in figure - the vibrational energy levels become nonequidistantly spaced for higher energies as the binding potential gets inharmonic - levels are more closely spaced at high energies as the potential well gets wider - selection rule for transitions between vibrational levels: - this rule can be found by calculating the dipole transition matrix element in the same way as discussed for the Hydrogen atom phys4.12 Page 15 Vibrational Spectra: - molecules can have different vibrational modes (normal modes), see figure - in complex molecules the modes can be local (affecting only part of the molecule) or global (affecting the whole molecule) - in water (H2O) bending modes have lower energy than stretching modes (this property is observed in many molecules) - different modes have different characteristic frequencies because of the different bond lengths and effective masses involved - analyzing vibrational spectra is a powerful tool to analyze the bonding in molecules, in particular if there could be different possible bond structures for a molecule of the same composition phys4.12 Page 16 Vibrational levels in molecules containing carbon (C): - the oscillation frequency of molecules with carbon-carbon bonds depend on the character of the bond - as expected multiple covalent bonds result in large spring constants k and larger frequencies ν0 vibrational levels in Carbon Dioxide: - symmetric stretch mode is not excited electromagnetically - other modes absorb infrared radiation which plays an important role in the warming of the earth's atmosphere (greenhouse effect) (also methane and water but not oxygen and nitrogen which have no optical modes) - symmetric bending mode: λ ~ 15 μm - asymmetric stretching mode: λ ~ 4.36 μm phys4.12 Page 17








