Chem 480A
advertisement

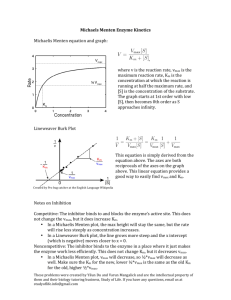
Chem 480A 3 Dec 99 41 Enzyme Kinetics The Michaelis-Menton mechanism (Maude Menton - graduated from U. Toronto, worked in U.S.) The Michaelis-Menton mechanism for enzyme kinetics is: E S k1 k2 ES E products . k 1 E is the enzyme, S is the “substrate” (the molecule on which the enzyme does its work), and ES is an enzyme-substrate complex. (It is presumed that the substrate must somehow bind to the enzyme before the enzyme can do its work.) We analyze this mechanism as usual. First, we define the reaction rate as the rate of formation of product and write the kinetic equation implied by this mechanism, d [product] k2 [ES] . dt The enzyme-substrate complex, ES, is a transient species so we set up an equation for its rate of change and apply the steady state approximation, d [ES] k1[E][S] k1[ES] k2[ES] 0 . dt Solve for [ES], [ES] k1[E][S] , k1 k2 and substitute it into the equation for the rate, Rate d [product] k [E][S] . k2 1 dt k1 k2 We might think that we are finished, but there is a complication and some new notation to introduce. First we introduce the Michaelis-Menton constant, KM, 1 k1 , K M k1 k2 so that the rate becomes, 1 Rate k2 [E][S] . KM Now we must deal with the difficulty that [E] is the concentration of free (uncomplexed) enzyme and this is usually not known. What is known is the total enzyme concentration, [E]o, but [E]o [E] [ES] [E] [E][S] KM [S] [E]1 KM from which we obtain, [E]o . [S] 1 KM [E] The rate becomes, then, k2 [E]o [S] KM [S] 1 KM . [E]o [S] k2 K M [S] Rate Define the reaction velocity as v = Rate. So, v k2 [E]o [S] . K M [S] Note that the reaction velocity, v, is zero when [S] is zero and that the reaction velocity increases as we increase [S]. The reaction velocity reaches a maximum when [S] becomes very large. Define the maximum velocity, vmax, as, vmax lim k2 [S] [E]o [S] k2 [E]o , K M [S] then v vmax [S] . K M [S] 2 Note that the kinetics of the reaction are characterized by two parameters, vmax and KM. In order to deal with experimental data we write, 1 KM 1 v vmax [S] vmax In an experiment one measures v as a function of [S]. If we plot 1/v against 1/[S] we should get a straight line with slope, KM/vmax and intercept 1/vmax. This gives us both parameters, K M slope vmax slope . intercept Enzyme With Inhibitor Recall the basic Michaelis-Menton mechanism, E S k1 k2 ES E products . k 1 There are several possibilities for an inhibitor, I, to interfere with this reaction: 1) E k '1 I EI . k '1 In words, the inhibitor binds with the enzyme to the exclusion of the substrate. 2) ES I k1" ESI . k" 1 In words, the inhibitor binds to the enzyme-substrate complex and alters the action of the enzyme on the substrate. You can have 1) or 2) or both. We will only work out the first case. The procedure is the same as for the uninhibited Michaelis-Menton mechanism except for an additional term in the expression for the total enzyme concentration and a new transient, EI. The rate is still d [product] k2 [ES] , dt and we apply the steady state approximation to ES, which leads to [ES] k1[E][S] , k1 k2 and the same rate expression 3 d [product] k [E][S] . k2 1 dt k1 k2 Rate Use the same definition of KM, 1 k1 , K M k1 k2 which leads to, Rate k2 [E][S] . KM But the enzyme-inhibitor complex is also a transient, d [EI] k '1[E][I] k '1[EI] 0 . dt giving [EI] k '1 [E][I] . k '1 Now the total enzyme concentration has an extra term [E]o [E] [ES] [EI] [E] [E][S] k '1 [E][I] KM k '1 [S] k '1 [E]1 [I] K M k '1 leading to [E] [E]o [S] k '1 [I] 1 K M k '1 and the rate is Rate k2 [E]o [S] . KM [S] k '1 [I] 1 K k ' M 1 4 vmax is still vmax k2 [E]o so the reaction velocity becomes vmax [S] v K M [S] K M k '1 [I] k '1 and then 1 KM 1 K k ' [I] . M 1 v vmax [S] vmax vmax k '1 [S] We still plot 1/v against 1/[S] and the intercept is 1/vmax, but the slope is slope K M K M k '1 [I] , vmax vmax k '1 and slope k' K M 1 1 [I] . intercept k '1 We must do several different experiments at different [I] to get KM and k'1/k'-1. Note that you can only get the ratio k'1/k'-1 and not the individual k's. 5